Comprehensive practical - answer key
Creating vectors/factors and data frames
- We are performing RNA-Seq on cancer samples being treated with three different types of treatment (A, B, and P). You have 12 samples total, with 4 replicates per treatment. Write the R code you would use to construct your metadata table as described below.
- Create the vectors/factors for each column (Hint: you can type out each vector/factor, or if you want the process go faster try exploring the
rep()function).
sex <- c("M", "F",...) # saved vectors/factors as variables and used c() or sex <- rep(c("M", "F"), times = 6) stage <- rep(c("I", "II", "II"), times = 4) treatment <- rep(c("A", "B", "P"), each = 4) myc <- c(2343,457,4593,9035,3450,3524,958,1053,8674,3424,463,5105)- Put them together into a dataframe called
meta.
meta <- data.frame(sex, stage, treatment, myc) # used data.frame() to create the table- Use the
rownames()function to assign row names to the dataframe (Hint: you can type out the row names as a vector, or if you want the process go faster try exploring thepaste()function).
rownames(meta) <- c("sample1", "sample2",... , "sample12") # or use: rownames(meta) <- paste("sample", 1:12, sep="")Your finished metadata table should have information for the variables
sex,stage,treatment, andmyclevels:sex stage treatment myc sample1 M I A 2343 sample2 F II A 457 sample3 M II A 4593 sample4 F I A 9035 sample5 M II B 3450 sample6 F II B 3524 sample7 M I B 958 sample8 F II B 1053 sample9 M II P 8674 sample10 F I P 3424 sample11 M II P 463 sample12 F II P 5105 - Create the vectors/factors for each column (Hint: you can type out each vector/factor, or if you want the process go faster try exploring the
Subsetting vectors/factors and dataframes
-
Using the
metadata frame from question #1, write out the R code you would use to perform the following operations (questions DO NOT build upon each other):- return only the
treatmentandsexcolumns using[]:
meta[ , c(1,3)]- return the
treatmentvalues for samples 5, 7, 9, and 10 using[]:
meta[c(5,7,9,10), 3]- remove the
treatmentcolumn from the dataset using[]:
meta[, -3]- remove samples 7, 8 and 9 from the dataset using
[]:
meta[-7:-9, ]- keep only samples 1-6 using
[]:
meta[1:6, ]- output a data frame with a column called
pre_treatmentas the first column with the values T, F, F, F, T, T, F, T, F, F, T, T (Hint: usedata.frameorcbind()):
pre_treatment <- c(T, F, F, F, T, T, F, T, F, F, T, T) cbind(pre_treatment, meta)- change the names of the columns to: “A”, “B”, “C”, “D”:
colnames(meta) <- c("A", "B", "C", "D") - return only the
Extracting components from lists
-
Create a new list,
list_threewith three components, thepre_treatmentvector, the dataframemeta, and vectorstage. Use this list to answer the questions below .list_threehas the following structure (NOTE: the components of this list are not currently named):[[1]] [1] 4.6 3000.0 50000.0 [[2]] species glengths 1 ecoli 4.6 2 human 3000.0 3 corn 50000.0 [[3]] [1] 8
list_three <- list(pre_treatment, meta, stage)
Write out the R code you would use to perform the following operations (questions DO NOT build upon each other):
- return the second component of the list:
list_three[[2]]
- return
2343from the second component of the list:
list_three[[2]][1,4]
#or
comp2 <- list_three[[2]]
comp2[1,4]
- return all “I” values from the third component:
list_three[[3]][c(1,4,7,10)]
#or
comp3 <- list_three[[3]]
comp3[c(1,4,7,10)]
- give the components of the list the following names: “pre_treatment”, “meta”, “stage”:
names(list_three) <- c("pre_treatment", "meta", "stage")
Creating figures with ggplot2
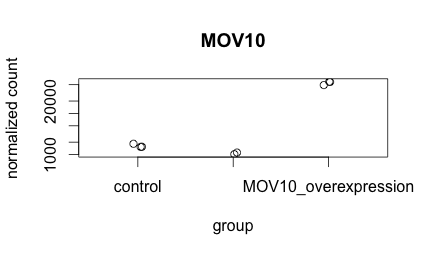
-
Create the same plot as above using ggplot2 using the provided metadata and counts datasets. The metadata table describes an experiment that you have setup for RNA-seq analysis, while the associated count matrix gives the normalized counts for each sample for every gene. Download the count matrix and metadata using the links provided.
Follow the instructions below to build your plot. Write the code you used and provide the final image.
-
Read in the metadata file (“Mov10_full_meta.txt”) to a variable called
metausing theread.delim()function. Be sure to specify the row names are in column 1 and the delimiter/column separator is a tab (“/t”).counts_meta <- read.delim("data/Mov10_full_meta.txt", sep="\t", row.names=1) -
Read in the count matrix file (“normalized_counts.txt”) to a variable called
datausing theread.delim()function and specifying there are row names in column 1 and the tab delimiter.data <- read.delim("data/normalized_counts.txt", sep="\t", row.names=1) -
Create a variable called
expressionthat contains the normalized count values from the row indatathat corresponds to theMOV10gene.
expression <- data["MOV10", ]- Check the class of
expression.data.frame
Convert this to a numeric vector using
as.numeric(expression)class(expression) expression <- as.numeric(expression) class(expression)- Bind that vector to your metadata data frame (
counts_meta) and call the new data framedf.
df <- cbind(counts_meta, expression) #or df <- data.frame(counts_meta, expression)-
Create a ggplot by constructing the plot line by line:
-
Initialize a ggplot with your
dfas input. -
Add the
geom_jitter()geometric object with the required aesthetics -
Color the points based on
sampletype -
Add the
theme_bw()layer -
Add the title “Expression of MOV10” to the plot
-
Change the x-axis label to be blank
-
Change the y-axis label to “Normalized counts”
-
Using
theme()change the following properties of the plot:-
Remove the legend (Hint: use ?theme help and scroll down to legend.position)
-
Change the plot title size to 1.5x the default and center align
-
Change the axis title to 1.5x the default size
-
Change the size of the axis text only on the y-axis to 1.25x the default size
-
Rotate the x-axis text to 45 degrees using
axis.text.x=element_text(angle=45, hjust=1)
-
ggplot(df) + geom_jitter(aes(x= sampletype, y= expression, color = sampletype)) + theme_bw() + ggtitle("Expression of MOV10") + xlab(NULL) + ylab("Normalized counts") + theme(legend.position = "none", plot.title=element_text(hjust=0.5, size=rel(1.5)), axis.text=element_text(size=rel(1.25)), axis.title=element_text(size=rel(1.5)), axis.text.x=element_text(angle=45, hjust=1)) -
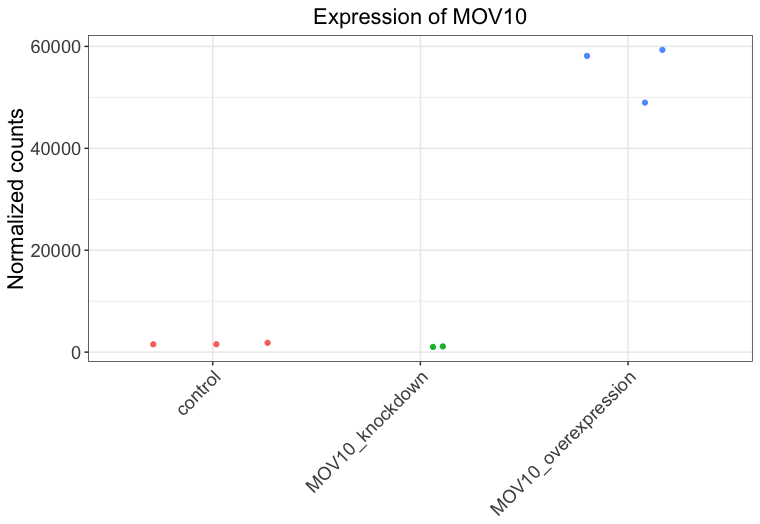
-
-
Save the plot as a PDF to the figures directory.
```r pdf(“figures/name_of_plot.pdf”)
ggplot(df) + geom_jitter(aes(x= sampletype, y= expression, color = sampletype)) + theme_bw() + ggtitle(“Expression of MOV10”) + xlab(NULL) + ylab(“Normalized counts”) + theme(legend.position = “none”, plot.title=element_text(hjust=0.5, size=rel(1.5)), axis.text=element_text(size=rel(1.25)), axis.title=element_text(size=rel(1.5)), axis.text.x=element_text(angle=45, hjust=1))
dev.off()
Packages and installations
- Install the
tidyverseR package from the CRAN repository and load the library.
install.packages("tidyverse")
library(tidyverse)
- Install the
biomaRtR package from the Bioconductor repository and load the library.
#install.packages("BiocManager")
library(BiocManager)
install("biomaRt")
library(biomaRt)
Building on the basics
The Tidyverse suite of integrated packages are R packages designed to work together to make common data science operations more user friendly. The packages have functions for data wrangling, tidying, reading/writing, parsing, and visualizing, among others.
-
Using this tutorial, explore some of the functionality for reading in and wrangling data with the
readranddplyrpackages, which were installed when you installed the tidyverse suite in the previous section. -
Turn the
metadata frame from question #1 of the “Creating vectors/factors and data frames” section above into a tibble calledmeta_tb. (Hint: Be sure to turn the rownames into a column before changing it into a tibble.)
meta_tb <- meta %>%
rownames_to_column(var = "sample") %>%
as_tibble()
- Using
meta_tb, write out the R code you would use to rename the columns back tosex,stage,treatment, andmycusingrename(). Save back (overwrite) to themeta_tbvariable.
meta_tb <- meta_tb %>%
rename(sex = A,
stage = B,
treatment = C,
myc = D)
-
Using
meta_tb, write out the R code you would use to perform the following operations (questions DO NOT build upon each other):- use
filter()to return all data for those samples receiving treatmentP.
meta_tb %>% filter(treatment == "P")- use
filter()/select()to return only thestageandtreatmentcolumns for those samples withmyc> 5000.
meta_tb %>% filter(myc > 5000) %>% dplyr::select(stage, treatment)- use
arrange()to arrange the rows bymycin descending order.
meta_tb %>% arrange(desc(myc)) - use
-
Write
meta_tbto file using thewrite_delim()function.write_delim(meta_tb, file = "results/metadata_myc_by_stage_sex.csv", delim = ",")