Learning Objectives:
- Automate a workflow by grouping a series of sequential commands into a script
- Modify and submit the workflow script to the cluster
Automating the analysis path from Sequence reads to Count matrix
Once you have optimized all the tools and parameters using a single sample, you can write a script to run the analysis on all the samples at the same time.
This will ensure that you run every sample with the exact same parameters, and will enable you to keep track of all the tools and their versions. In addition, the script is like a lab notebook, in the future you (or your colleagues) can go back and check the workflow for methods, which enables efficiency and reproducibility.
Before we start with the script, let’s check how many cores our interactive session has by using bjobs.
$ bjobs
If you already have an interactive session with 2 or more cores, then do not start a new interactive session. If you don’t, then exit out of the one you have and run the following command.
$ bsub -Is -n 2 -q interactive bash
More Flexibility with variables
We can write a shell script that will run on a specific file, but to make it more flexible and efficient we would prefer that it lets us give it an input fastq file when we run the script. To be able to provide an input to any shell script, we need to use so-called Positional Parameters.
For example, we can refer to the components of the following command as numbered variables within the actual script:
# * DO NOT RUN *
sh run_rnaseq.sh input.fq input.gtf 12
$0 => run_rnaseq.sh
$1 => input.fq
$2 => input.gtf
$3 => 12
The variables $1, $2, $3,…$9 and so on are positional parameters in the context of the shell script, and can be used within the script to refer to the files/number specified on the command line. Basically, the script is written with the expectation that $1 will be a fastq file and $2 will be a GTF file, and so on.
There can be virtually unlimited numbers of inputs to a shell script, but it is wise to only have a few inputs to avoid errors and confusion when running a script that used positional parameters.
This is an example of a simple script that used the concept of positional parameters and the associated variables. You should try this script out after the class to get a better handle on positional parameters for shell scripting.
Let’s use this new concept we have just learned in the script we are writing. We want the first positional parameter ($1) to be the name of our fastq file. We could just use the variable $1 throughout the script to refer to the fastq file, but this variable name is not intuitive, so we want to create a new variable called fq and copy the contents of $1 into it.
#!/bin/bash/
# initialize a variable with an intuitive name to store the name of the input fastq file
fq=$1
When we set up variables we do not use the
$before it, but when we use the variable, we always have to have the$before it. >For example:
initializing the
fqvariable =>fq=$1using the
fqvariable =>fastqc $fq
To ensure that all the output files from the workflow are properly named with sample IDs we should extract the “base name” (or sample ID) from the name of the input file.
# grab base of filename for naming outputs
base=$(basename $fq .subset.fq)
echo "Sample name is $base"
Remember
basename?
- the
basenamecommand: this command takes a path or a name and trims away all the information before the last\and if you specify the string to clear away at the end, it will do that as well. In this case, if the variable$fqcontains the path ”~/unix_lesson/rnaseq/raw_data/Mov10_oe_1.subset.fq”,basename $fq .subset.fqwill output “Mov10_oe_1”.- to assign this value to the
basevariable, we place thebasename...command in parentheses and put a$outside. This syntax is necessary for assigning the output of a command to a variable.
Next we want to specify how many cores the script should use to run the analysis. This provides us with an easy way to modify the script to run with more or fewer cores without have to replace the number within all commands where cores are specified.
# specify the number of cores to use
cores=2
Next we’ll initialize 2 more variables named genome and gtf, these will contain the paths to where the reference files are stored. This makes it easier to modify the script for when you want to use a different genome, i.e. you’ll just have to change the contents of these variable at the beginning of the script.
# directory with genome reference FASTA and index files + name of the gene annotation file
genome=/groups/hbctraining/intro_rnaseq_hpc/reference_STAR/
gtf=~/unix_lesson/rnaseq/reference_data/chr1-hg19_genes.gtf
We’ll create output directories, but with the -p option. This will make sure that mkdir will create the directory only if it does not exist, and it won’t throw an error if it does exist.
# make all of the output directories
# The -p option means mkdir will create the whole path if it
# does not exist and refrain from complaining if it does exist
mkdir -p ~/unix_lesson/rnaseq/results/fastqc/
mkdir -p ~/unix_lesson/rnaseq/results/STAR
mkdir -p ~/unix_lesson/rnaseq/results/counts
Now that we have already created our output directories, we can now specify variables with the path to those directories both for convenience but also to make it easier to see what is going on in a long command.
# set up output filenames and locations
fastqc_out=~/unix_lesson/rnaseq/results/fastqc/
align_out=~/unix_lesson/rnaseq/results/STAR/${base}_
counts_input_bam=~/unix_lesson/rnaseq/results/STAR/${base}_Aligned.sortedByCoord.out.bam
counts=~/unix_lesson/rnaseq/results/counts/${base}_featurecounts.txt
Keeping track of tool versions
All of our variables are now staged. Next, let’s make sure all the modules are loaded. This is also a good way to keep track of the versions of tools that you are using in the script:
# set up the software environment
module load seq/fastqc/0.11.3
module load seq/STAR/2.5.3a
module load seq/samtools/1.3
PATH=/opt/bcbio/centos/bin:$PATH # for using featureCounts if not already in $PATH
Preparing for future debugging
In the script, it is a good idea to use echo for debugging. echo basically displays the string of characters specified within the quotations. When you have strategically place echo commands specifying what stage of the analysis is next, in case of failure you can determine the last echo statement displayed to troubleshoot the script.
echo "Processing file $fq"
You can also use
set -x:
set -xis a debugging tool that will make bash display the command before executing it. In case of an issue with the commands in the shell script, this type of debugging lets you quickly pinpoint the step that is throwing an error. Often, tools will display the error that caused the program to stop running, so keep this in mind for times when you are running into issues where this is not available. You can turn this functionality off by sayingset +x
Running the tools
# Run FastQC and move output to the appropriate folder
fastqc $fq
# Run STAR
STAR --runThreadN $cores --genomeDir $genome --readFilesIn $fq --outFileNamePrefix $align_out --outFilterMultimapNmax 10 --outSAMstrandField intronMotif --outReadsUnmapped Fastx --outSAMtype BAM SortedByCoordinate --outSAMunmapped Within --outSAMattributes NH HI NM MD AS
# Create BAM index
samtools index $counts_input_bam
# Count mapped reads
featureCounts -T $cores -s 2 -a $gtf -o $counts $counts_input_bam
Last addition to the script
It is best practice to have the script usage specified at the top any script. This should have information such that when your future self, or a co-worker, uses the script they know what it will do and what input(s) are needed. For our script, we should have the following lines of comments right at the top after #!/bin/bash/:
# This script takes a fastq file of RNA-Seq data, runs FastQC and outputs a counts file for it.
# USAGE: sh rnaseq_analysis_on_allfiles.sh <name of fastq file>
It is okay to specify this everything else is set up, since you will have most clarity about the script only once it is fully done.
Saving and running script
To transfer the contents of the script to Orchestra, you can copy and paste the contents into a new file using nano.
$ cd ~/unix_lesson/rnaseq/scripts/
$ nano rnaseq_analysis_on_input_file.sh
Alternatively, you can save the script on your computer and transfer it to ~/unix_lesson/rnaseq/scripts/ using FileZilla.
We should all have an interactive session with 2 or more cores, so we can start the script:
$ sh rnaseq_analysis_on_input_file.sh ~/unix_lesson/rnaseq/raw_data/Mov10_oe_1.subset.fq
Before we move to the next section, modify the number of cores in the above script to 6 using nano, so we can have it run a lot faster when we submit it as an LSF job.
Running the script iteratively as a job submission to the LSF scheduler
The above script will run in an interactive session one file at a time. But the whole point of writing this script was to run it on all files at once. How do you think we can do this?
To run the above script “in serial” for all of the files on a worker node via the job scheduler, we can create a separate submission script that will need 2 components:
- LSF directives at the beginning of the script. This is so that the scheduler knows what resources we need in order to run our job on the compute node(s).
- a
forloop that iterates through and runs the above script for all the fastq files.
Below is what this second script would look like [DO NOT RUN THIS]:
#!/bin/bash
#BSUB -q training # Partition to submit to (comma separated)
#BSUB -n 6 # Number of cores, since we are running the STAR and featureCounts commands with 6 threads
#BSUB -W 1:30 # Runtime in D-HH:MM (or use minutes)
#BSUB -R "rusage[mem=4000]" # Memory in MB
#BSUB -J rnaseq_mov10 # Job name
#BSUB -o %J.out # File to which standard output will be written
#BSUB -e %J.err # File to which standard error will be written
# this `for` loop, will take the fastq files as input and run the script for all of them one after the other.
for fq in ~/unix_lesson/rnaseq/raw_data/*.fq
do
echo "running analysis on $fq"
rnaseq_analysis_on_input_file.sh $fq
done
But we don’t want to run the analysis on these 6 samples one after the other! We want to run them “in parallel” as 6 separate jobs.
Note: If you create and run the above script, or something similar to it, i.e. with LSF directives at the top, you should give the script name .lsf as the extension. This will make it obvious that it is meant to submit jobs to the LSF scheduler.
Exercise
How would you run the above script?
Parallelizing the analysis for efficiency
Parallelization will save you a lot of time with real (large) datasets. To parallelize our analysis, we will still need to write a second script that will call the original script we just wrote. We will still use a for loop, but we will be creating a regular shell script and we will be specifying the LSF directives differently.
Use nano to start a new shell script called rnaseq_analysis_on_allfiles-for_lsf.sh:
$ nano rnaseq_analysis_on_allfiles_for-lsf.sh
This script loops through the same files as in the previous (demo) script, but the command being submitted within the for loop is bsub with LSF directives specified on the same line:
#! /bin/bash
for fq in ~/unix_lesson/rnaseq/raw_data/*.fq
do
bsub -q training -n 6 -W 1:30 -R "rusage[mem=4000]" -J rnaseq_mov10 -o %J.out -e %J.err "sh ~/unix_lesson/rnaseq/scripts/rnaseq_analysis_on_input_file.sh $fq"
sleep 1 # wait 1 second between each job submission
done
Please note that after the bsub directives the command sh ~/unix_lesson/rnaseq/scripts/rnaseq_analysis_on_input_file.sh $fq is in quotes.
What you should see on the output of your screen would be the jobIDs that are returned from the scheduler for each of the jobs that your script submitted.
You can use the bjobs command to check progress (though there is a lag of about 60 seconds between what is happening and what is reported).
Don’t forget about the bkill command, should something go wrong and you need to cancel your jobs.
NOTE: All job schedulers are similar, but not the same. Once you understand how one works, you can transition to another one without too much trouble. They all have their pros and cons which are considered by the system administrators when picking one for a given HPC environment.
This fall, HMS-RC is introducing a new cluster called O2 (Orchestra 2), which used the SLURM scheduler instead of LSF.
Generating a Count Matrix
The above script will generate separate count files for each sample. Hence, after the script has run, you will have to merge them using paste and do some cleanup using cut to generate a full count matrix wherein the first column is gene names and the rest of the columns are gene counts in each sample (as shown below).
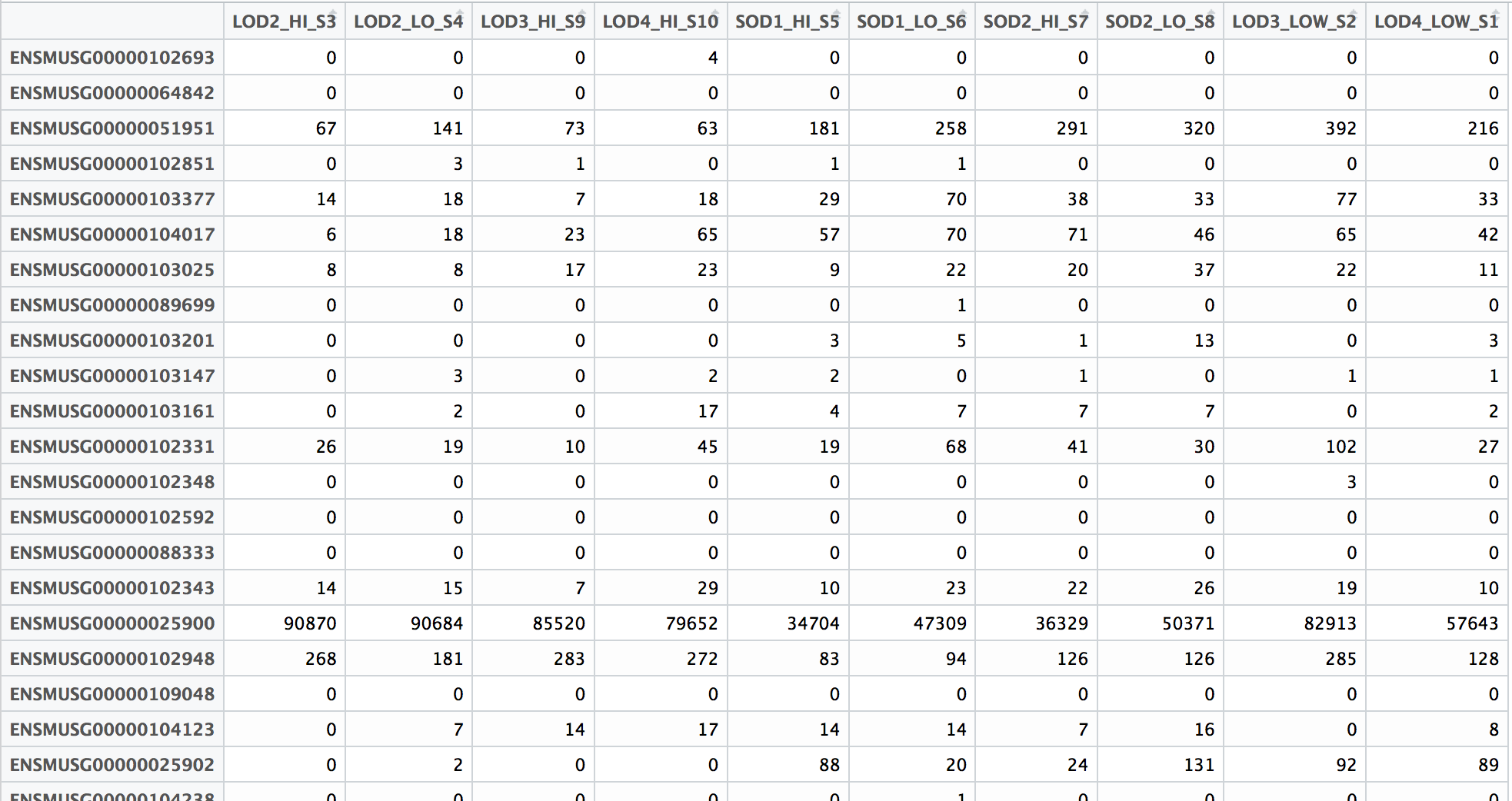
Alternatively, you could remove featureCounts from the original script, and run it after all the jobs finish generating the BAM files.
This lesson has been developed by members of the teaching team at the Harvard Chan Bioinformatics Core (HBC). These are open access materials distributed under the terms of the Creative Commons Attribution license (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- The materials used in this lesson was derived from work that is Copyright © Data Carpentry (http://datacarpentry.org/). All Data Carpentry instructional material is made available under the Creative Commons Attribution license (CC BY 4.0).