Approximate time: 110 min
Learning Objectives
- Implement matching and re-ordering data within data structures.
Matching data
Often when working with genomic data, we have a data file that corresponds with our metadata file. The data file contains measurements from the biological assay for each individual sample. In this case, the biological assay is gene expression and data was generated using RNA-Seq.
Let’s read in our expression data (RPKM matrix) that we downloaded previously:
rpkm_data <- read.csv("data/counts.rpkm.csv")
NOTE: If the data file name ends with
txtinstead ofcsv, you can read in the data using the code:rpkm_data <- read.csv("data/counts.rpkm.txt").
Take a look at the first few lines of the data matrix to see what’s in there.
head(rpkm_data)
It looks as if the sample names (header) in our data matrix are similar to the row names of our metadata file, but it’s hard to tell since they are not in the same order. We can do a quick check of the number of columns in the count data and the rows in the metadata and at least see if the numbers match up.
ncol(rpkm_data)
nrow(metadata)
What we want to know is, do we have data for every sample that we have metadata?
The %in% operator
Although lacking in documentation this operator is well-used and convenient once you get the hang of it. The operator is used with the following syntax:
vector1_of_values %in% vector2_of_values
It will take a vector as input to the left and will evaluate each element to see if there is a match in the vector that follows on the right of the operator. The two vectors do not have to be the same size. This operation will return a vector of the same length as vector1 containing logical values to indicate whether or not there was a match. Take a look at the example below:
A <- c(1,3,5,7,9,11) # odd numbers
B <- c(2,4,6,8,10,12) # even numbers
# test to see if each of the elements of A is in B
A %in% B
## [1] FALSE FALSE FALSE FALSE FALSE FALSE
Since vector A contains only odd numbers and vector B contains only even numbers, there is no overlap and so the vector returned contains a FALSE for each element. Let’s change a couple of numbers inside vector B to match vector A:
A <- c(1,3,5,7,9,11) # odd numbers
B <- c(2,4,6,8,1,5) # add some odd numbers in
# test to see if each of the elements of A is in B
A %in% B
## [1] TRUE FALSE TRUE FALSE FALSE FALSE
The logical vector returned denotes which elements in A are also in B and which are not.
We saw previously that we could use the output from a logical expression to subset data by returning only the values corresponding to TRUE. Therefore, we can use the output logical vector to subset our data, and return only those elements in A, which are also in B by returning only the TRUE values:
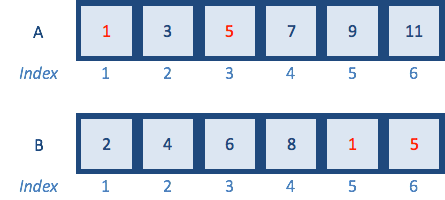
intersection <- A %in% B
intersection
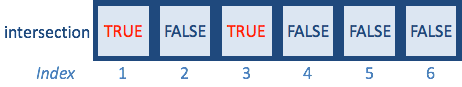
A[intersection]

In these previous examples, the vectors were small and so it’s easy to count by eye; but when we work with large datasets this is not practical. A quick way to assess whether or not we had any matches would be to use the any function to see if any of the values contained in vector A are also in vector B:
any(A %in% B)
The all function is also useful. Given a logical vector, it will tell you whether all values returned are TRUE. If there is at least one FALSE value, the all function will return a FALSE and you know that all of A are not contained in B.
all(A %in% B)
-
Using the
AandBvectors created above, evaluate each element inBto see if there is a match inA -
Subset the
Bvector to only return those values that are also inA.
Suppose we had two vectors that had the same values but just not in the same order. We could also use all to test for that. Rather than using the %in% operator we would use == and compare each element to the same position in the other vector. Unlike the %in% operator, for this to work you must have two vectors that are of equal length.
A <- c(10,20,30,40,50)
B <- c(50,40,30,20,10) # same numbers but backwards
# test to see if each element of A is in B
A %in% B
# test to see if each element of A is in the same position in B
A == B
# use all() to check if they are a perfect match
all(A == B)
Let’s try this on our data and see whether we have metadata information for all samples in our expression data. We’ll start by creating two vectors; one with the rownames of the metadata and colnames of the RPKM data. These are base functions in R which allow you to extract the row and column names as a vector:
x <- rownames(metadata)
y <- colnames(rpkm_data)
Now check to see that all of x are in y:
all(x %in% y)
Note that we can use nested functions in place of x and y:
all(rownames(metadata) %in% colnames(rpkm_data))
We know that all samples are present, but are they in the same order:
all(rownames(metadata) == colnames(rpkm_data))
Looks like all of the samples are there, but will need to be reordered. To reorder our genomic samples, we need to first learn different ways to reorder data. Therefore, we will step away from our genomic data briefly to learn about reordering, then return to it at the end of this lesson.
We have a list of 6 marker genes that we are very interested in. Our goal is to extract count data for these genes using the %in% operator from the rpkm_data data frame, instead scrolling through rpkm_data and finding them.
First, let’s create a vector called important_genes with the Ensembl IDs of the 6 genes we are interested in:
important_genes <- c("ENSMUSG00000083700", "ENSMUSG00000080990", "ENSMUSG00000065619", "ENSMUSG00000047945", "ENSMUSG00000081010", "ENSMUSG00000030970")
-
Use the
%in%operator to determine if all of these genes are in the row names of therpkm_datadata frame. -
Extract the rows from
rpkm_datathat correspond to these 6 genes using[]and the%in%operator. Double check the row names to ensure that you are extracting the correct rows. -
Bonus question: Extract the rows from
rpkm_datathat correspond to these 6 genes using[], but without using the%in%operator.
Reordering data using indices
Indexing [ ] can be used to extract values from a dataset as we saw earlier, but we can also use it to rearrange our data values.
teaching_team <- c("Jihe", "Mary", "Meeta", "Radhika")
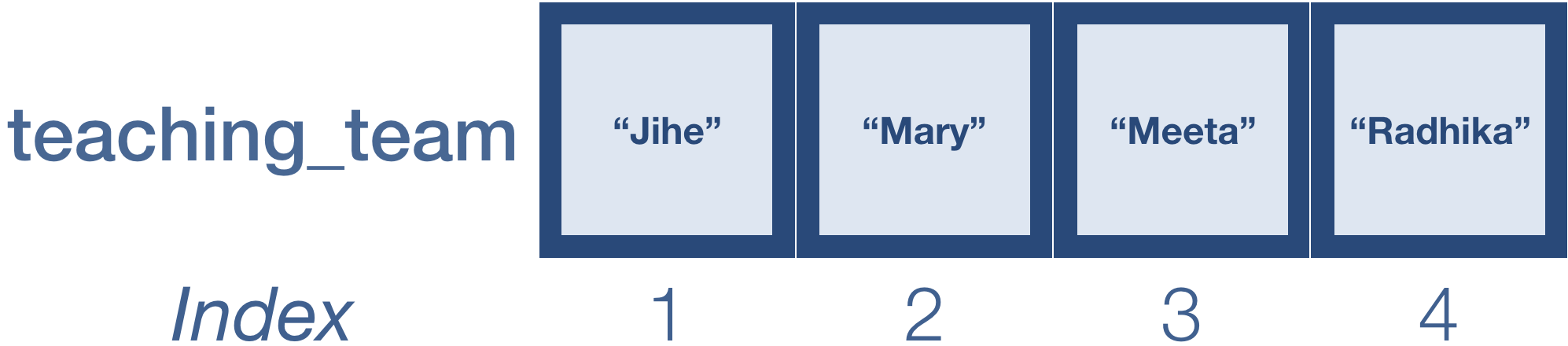
Remember that we can return values in a vector by specifying it’s position or index:
teaching_team[c(2, 4)] # Extracting values from a vector
teaching_team
We can also extract the values and reorder them:
teaching_team[c(4, 2)] # Extracting values and reordering them
Similarly, we can extract all of the values and reorder them:
teaching_team[c(4, 2, 1, 3)]
If we want to save our results, we need to assign to a variable:
reorder_teach <- teaching_team[c(4, 2, 1, 3)] # Saving the results to a variable
The match function
Now that we know how to reorder using indices, we can use the match() function to match the values in two vectors. We’ll be using it to evaluate which samples are present in both our counts and metadata dataframes, and then to re-order the columns in the counts matrix to match the row names in the metadata matrix.
match() takes at least 2 arguments:
- a vector of values in the order you want
- a vector of values to be reordered
The function returns the position of the matches (indices) with respect to the second vector, which can be used to re-order it so that it matches the order in the first vector. Let’s create vectors first and second to demonstrate how it works:
first <- c("A","B","C","D","E")
second <- c("B","D","E","A","C") # same letters but different order
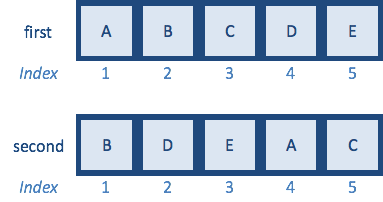
How would you reorder second vector to match first using indices?
If we had large datasets, it would be difficult to reorder them by searching for the indices of the matching elements. This is where the match function comes in really handy:
match(first,second)
[1] 4 1 5 2 3
The function should return a vector of size length(first). Each number that is returned represents the index of the second vector where the matching value was observed.
Now, we can just use the indices to reorder the elements of the second vector to be in the same positions as the matching elements in the first vector:
reorder_idx <- match(first,second) # Saving indices for how to reorder `second` to match `first`
second[reorder_idx] # Reordering the second vector to match the order of the first vector
second_reordered <- second[reorder_idx] # Reordering and saving the output to a variable
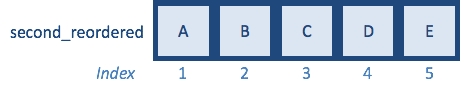
Now that we know how match() works, let’s change vector second so that only a subset are retained:
first <- c("A","B","C","D","E")
second <- c("D","B","A") # remove values
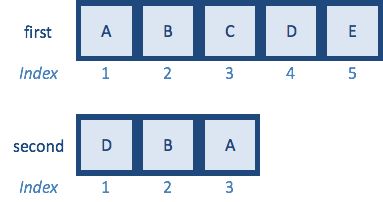
And try to match() again:
match(first,second)
[1] 3 2 NA 1 NA
NOTE: For values that don’t match by default return an
NAvalue. You can specify what values you would have it assigned usingnomatchargument. Also, if there is more than one matching value found only the first is reported.
Reordering genomic data using match() function
Using the match function, we now would like to match the row names of our metadata to the column names of our expression data*, so these will be the arguments for match. Using these two arguments we will retrieve a vector of match indices. The resulting vector represents the re-ordering of the column names in our data matrix to be identical to the rows in metadata:
rownames(metadata)
colnames(rpkm_data)
genomic_idx <- match(rownames(metadata), colnames(rpkm_data))
genomic_idx
Now we can create a new data matrix in which columns are re-ordered based on the match indices:
rpkm_ordered <- rpkm_data[,genomic_idx]
Check and see what happened by using head. You can also verify that column names of this new data matrix matches the metadata row names by using the all function:
head(rpkm_ordered)
all(rownames(metadata) == colnames(rpkm_ordered))
Now that our samples are ordered the same in our metadata and counts data, if these were raw counts we could proceed to perform differential expression analysis with this dataset.
This lesson has been developed by members of the teaching team at the Harvard Chan Bioinformatics Core (HBC). These are open access materials distributed under the terms of the Creative Commons Attribution license (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.