Approximate time: 80 minutes
Learning Objectives
- Generate bigWig files
- Visualizing enrichment patterns at particular locations in the genome
Visualization of ChIP-seq data
The first part of ChIP-sequencing analysis uses common processing pipelines, which involves the alignment of raw reads to the genome, data filtering, and identification of enriched signal regions (peak calling). In the second stage, individual software programs allow detailed analysis of those peaks, biological interpretation, and visualization of ChIP-seq results.
There are various strategies for visualizing enrichment patterns and we will explore a few of them. To start, we will create bigWig files for our samples, a standard file format commonly used for ChIP-seq data visualization.
Creating bigWig files
The first thing we want to do is take our alignment files (BAM) and convert them into bigWig files. The bigWig format is an indexed binary format useful for dense, continuous data that will be displayed in a genome browser as a graph/track, but also is used as input for some of the visualization commands we will be running in deepTools.
deepTools, is a suite of Python tools developed for the efficient analysis of high-throughput sequencing data, such as ChIP-seq, RNA-seq or MNase-seq. deepTools has a wide variety of commands that go beyond those that are covered in this lesson. We encourage you to look through the docuementation and explore on your own time.
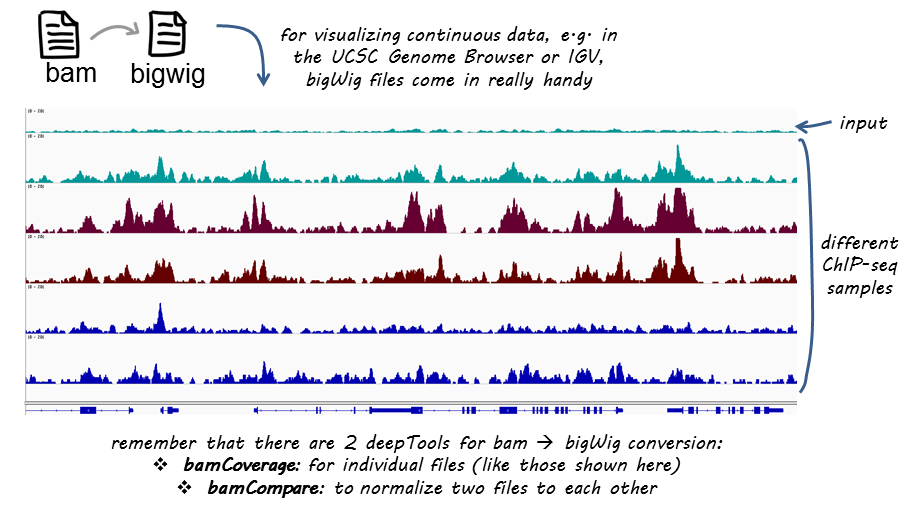
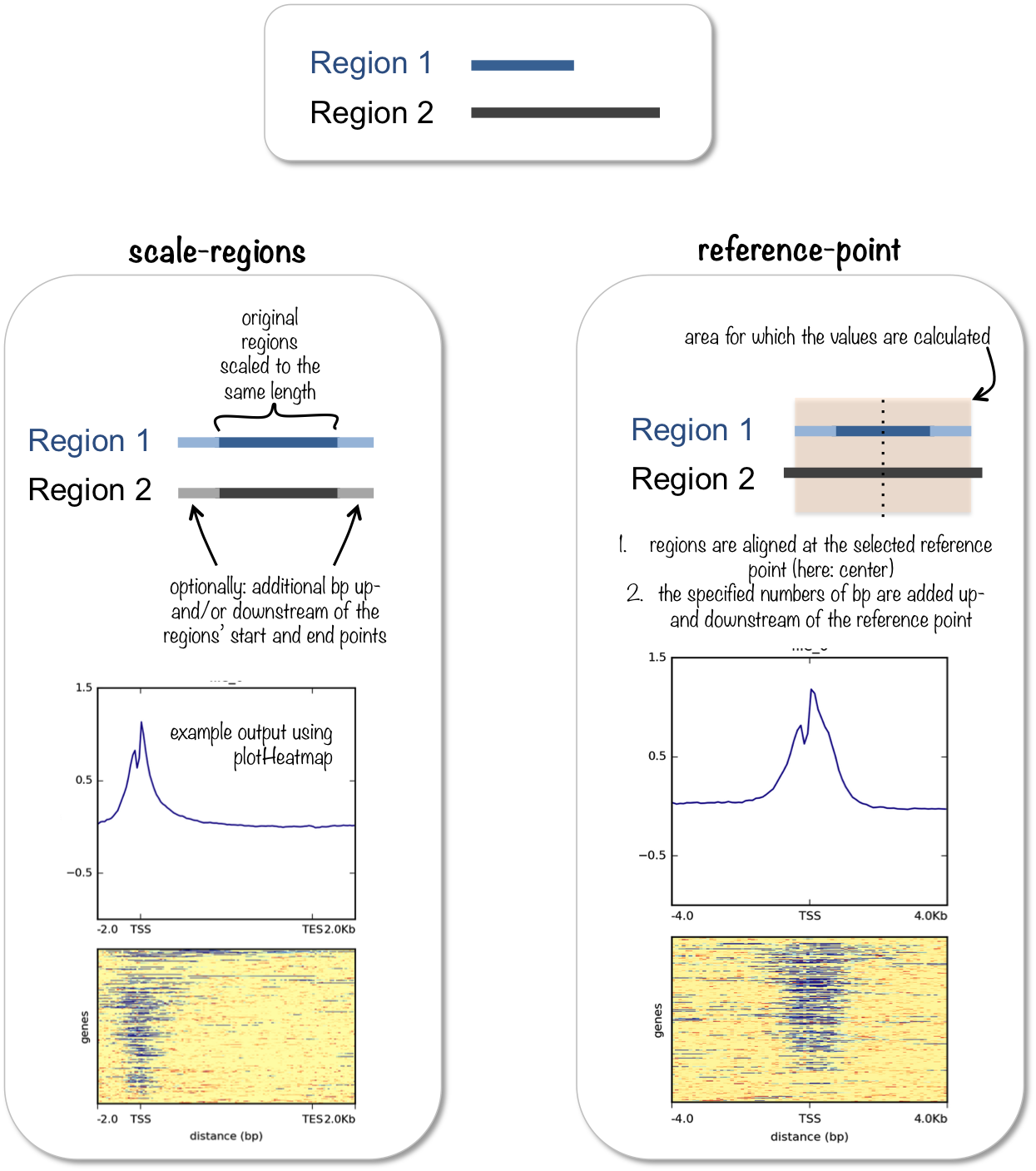
Image acquired from deepTools documentation pages
Start an interactive session with 6 cores. If you are already logged on to a compute node you will want to exit and start a new session.
$ srun --pty -p interactive -t 0-12:00 --mem 8G -c 6 --reservation=HBC2 bash
We will begin by creating a directory for the visualization output and loading the required modules to run deepTools.
$ cd ~/chipseq/results/
$ mkdir -p visualization/bigWig visualization/figures
$ module load gcc/6.2.0 python/2.7.12
$ module load deeptools/3.0.2
One last thing we need to do is create an index file for each one of our BAM files. To perform some functions on the BAM file, many tools require an index. Think of an index located at the back of a textbook. When you are interested in a particular subject area you look for the keyword in the index and identify the pages that contain the relevant information. Similarily, indexing the BAM file aims to achieve fast retrieval of alignments overlapping a specified region without going through the whole alignment file.
In order to index a BAM file, we will use SAMtools, a tool suite that provides alot of functionality in dealing with alignment files. There is a command called samtools index, which is what we will use. Since we need an index for each of our BAM files, we will put this in a for loop to avoid having to run the same command multiple times.
First, let’s load the module:
$ module load samtools/1.9
Now, at the command prompt start the for loop:
for file in ~/chipseq/results/bowtie2/*aln.bam
do
samtools index $file
done
NOTE: The above is assuming that you are pressing return after each line of code. If you wanted you could also run this command as a single line:
$ for file in ~/chipseq/results/bowtie2/*aln.bam; do samtools index $file; done
Now, to create our bigWig files there are two tools that can be useful: bamCoverage and bamCompare. The former will take in a single BAM file and return to you a bigWig file. The latter allows you to normalize two files to each other (i.e. ChIP sample relative to input) and will return a single bigWig file.
Let’s create a bigWig file for Nanog replicate 2 using the bamCoverage command. In addition to the input and output files, there are a few additional parameters we have added.
normalizeUsing: Possible choices: RPKM, CPM, BPM, RPGC. We will use BPM (Bins Per Million), which is similar to TPM in RNA-seq. BPM (per bin) = number of reads per bin / sum of all reads per bin (in millions).binSize: size of bins in basessmoothLength: defines a window, larger than thebinSize, to average the number of reads over. This helps produce a more continuous plot.centerReads: reads are centered with respect to the fragment length as specified byextendReads. This option is useful to get a sharper signal around enriched regions.
$ bamCoverage -b bowtie2/H1hesc_Nanog_Rep2_aln.bam \
-o visualization/bigWig/H1hesc_Nanog_Rep2.bw \
--binSize 20 \
--normalizeUsing BPM \
--smoothLength 60 \
--extendReads 150 \
--centerReads \
-p 6 2> ../logs/Nanog_rep2_bamCoverage.log
We can do the same for the Pou5f1 replicate 1:
$ bamCoverage -b bowtie2/H1hesc_Pou5f1_Rep1_aln.bam \
-o visualization/bigWig/H1hesc_Pou5f1_Rep1.bw \
--binSize 20 \
--normalizeUsing BPM \
--smoothLength 60 \
--extendReads 150 \
--centerReads \
-p 6 2> ../logs/Pou5f1_rep1_bamCoverage.log
NOTE: There is a reason we chose the specific replicates for the above commands, and it will become more obvious as we get to the end of this lesson!
Now, if we wanted to create a bigWig file in which we normalize the ChIP against the input we would use bamCompare. The command is quite similar to bamCoverage, the only difference being you require two files as input (b1 and b2).
## DO NOT RUN THIS
$ bamCompare -b1 bowtie2/H1hesc_Pou5f1_Rep1_aln.bam \
-b2 bowtie2/H1hesc_Input_Rep1_chr12_aln.bam \
-o visualization/bigWig/H1hesc_Pou5f1_Rep1_bgNorm.bw \
--binSize 20 \
--normalizeUsing BPM \
--smoothLength 60 \
--extendReads 150 \
--centerReads \
-p 6 2> ../logs/Pou5f1_rep1_bamCompare.log
NOTE: When you are creating bigWig files for your full dataset, this will take considerably longer and you will not want to run this interactively (except for testing purposes). Instead, you will want to write a job submission script with a loop that runs this command over all of your BAM files.
Since we are using a toy dataset which contains only a subset of the data, using these bigWigs for visualization would not give us meaningful results. As such, we have created bigWig files from the full dataset that you can use for the rest of this lesson.
Profile plots and heatmaps
Because many cis-regulatory elements are close to TSSs of their targets, a common visualization technique is to use bigWig files to obtain a global evaluation of enrichment around the TSS. In our example, we will assess enrichment around the TSS and plot this separately for the Nanog and Pou5f1 samples (two replicates in each plot).
Rather than looking at the TSS for all known genes, we will only look be looking at genes on chromosome 12 in the interest of time. Copy over the BED file which contains the coordinates for all genes on chromosome 12 to the visualization folder.
$ cp /n/groups/hbctraining/chip-seq/deepTools/chr12_genes.bed ~/chipseq/results/visualization/
Before we start plotting our data, we first need to prepare an intermediate file that can be used with the plotHeatmap and plotProfile commands.
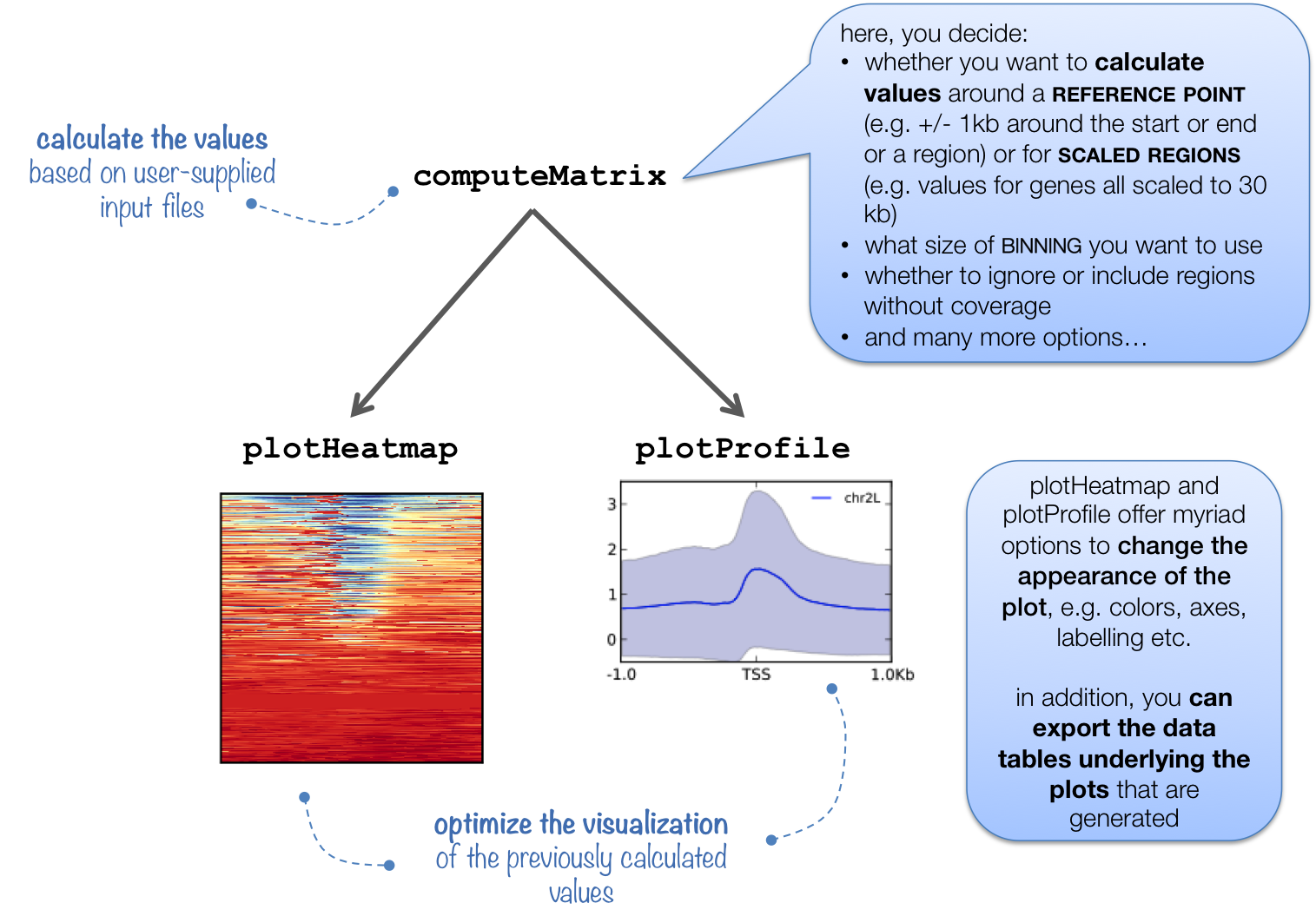
The computeMatrix command accepts multiple bigWig files and multiple region files (BED format) to create a count matrix which is the intermediate file. It can also be used to filter and sort regions according to their score. Our region file will be the BED file we just copied over and our bigWig files will be those generated from the full dataset that we have provided for you. Additionally, we will specify a window of +/- 1000bp around the TSS of genes (-b and -a). For each window, computeMatrix will calculate scores based on the read density values in the bigWig files.
First, let’s create a matrix for one of the Nanog replicates:
$ computeMatrix reference-point --referencePoint TSS \
-b 1000 -a 1000 \
-R ~/chipseq/results/visualization/chr12_genes.bed \
-S /n/groups/hbctraining/chip-seq/full-dataset/bigWig/Encode_Nanog*.bw \
--skipZeros \
-o ~/chipseq/results/visualization/matrixNanog_TSS_chr12.gz \
-p 6 \
--outFileSortedRegions ~/chipseq/results/visualization/regions_TSS_chr12.bed
NOTE: Typically, the genome regions are genes, and can be obtained from the UCSC table browser. Alternatively, you could look at other regions of interest that are not genomic feature related (i.e. binding regions from another protein of interest).
Now, let’s create another matrix for the Pou5f1 replicates:
$ computeMatrix reference-point --referencePoint TSS \
-b 1000 -a 1000 \
-R ~/chipseq/results/visualization/chr12_genes.bed \
-S /n/groups/hbctraining/chip-seq/full-dataset/bigWig/Encode_Pou5f1*.bw \
--skipZeros \
-p 6 \
-o ~/chipseq/results/visualization/matrixPou5f1_TSS_chr12.gz \
--outFileSortedRegions ~/chipseq/results/visualization/regionsPou5f1_TSS_chr12.bed
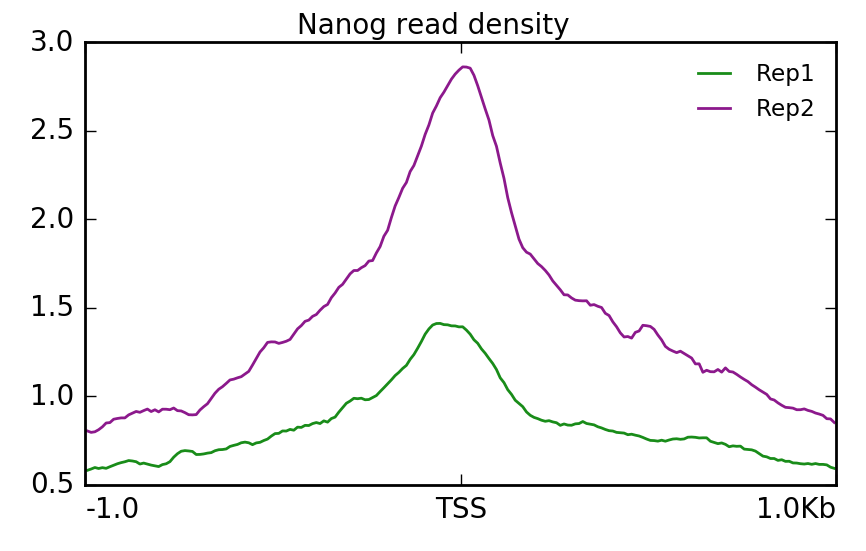
Using that matrix we can create a profile plot which is essentially a density plot that evaluates read density across all transcription start sites. For Nanog, we can see that Replicate 2 has a particularly higher amount of signal at the TSS compared to Replicate 1.
$ plotProfile -m visualization/matrixNanog_TSS_chr12.gz \
-out visualization/figures/TSS_Nanog_profile.png \
--perGroup \
--colors green purple \
--plotTitle "" --samplesLabel "Rep1" "Rep2" \
--refPointLabel "TSS" \
-T "Nanog read density" \
-z ""
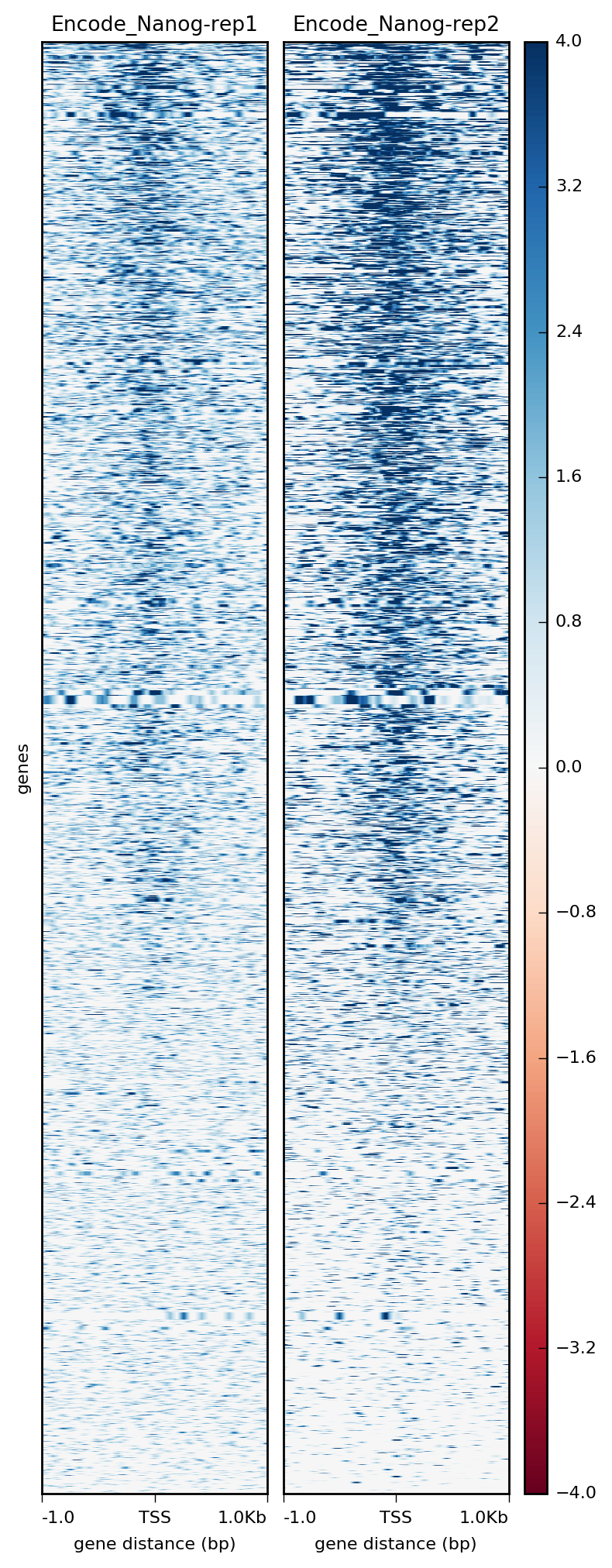
Alternatively, we could use a heatmap to evaluate the same matrix of information:
$ plotHeatmap -m visualization/matrixNanog_TSS_chr12.gz \
-out visualization/figures/TSS_Nanog_heatmap.png \
--colorMap RdBu \
--whatToShow 'heatmap and colorbar' \
--zMin -4 --zMax 4
Similarly we can do the same for Pou5f1. Here, we find that Replicate 1 exhibits stronger signal.
$ plotProfile -m visualization/matrixPou5f1_TSS_chr12.gz \
-out visualization/figures/TSS_Pou5f1_profile.png \
--perGroup --colors green purple \
--plotTitle "" --samplesLabel "Rep1" "Rep2" \
--refPointLabel "TSS" -T "Pou5f1 read density" -z ""
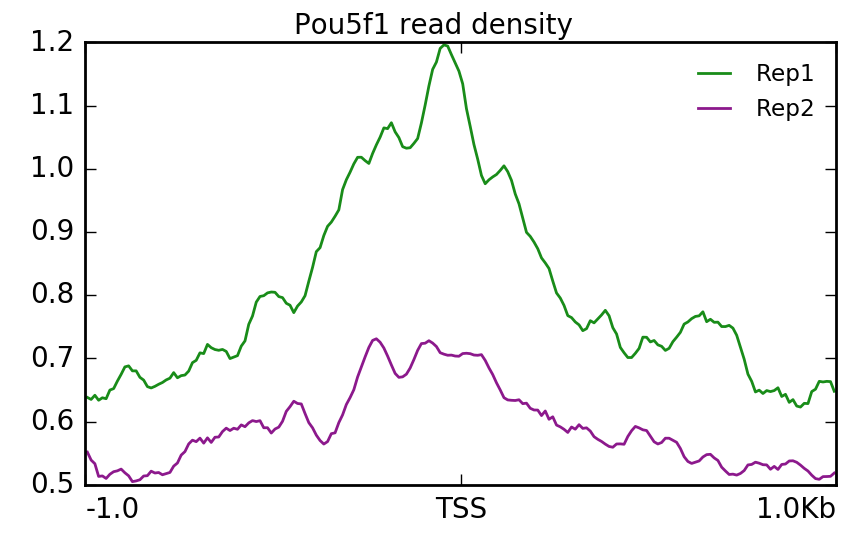
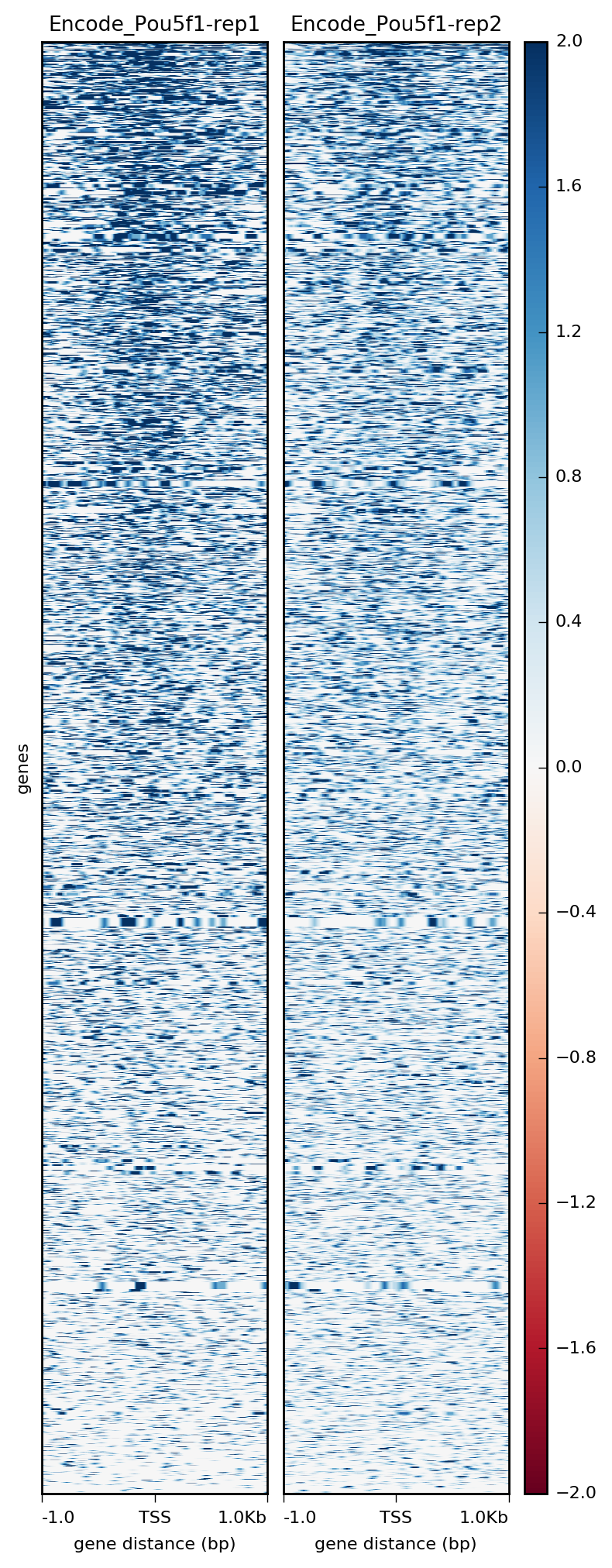
$ plotHeatmap -m visualization/matrixPou5f1_TSS_chr12.gz \
-out visualization/figures/TSS_Pou5f1_heatmap.png \
--colorMap RdBu \
--whatToShow 'heatmap and colorbar' \
--zMin -2 --zMax 2
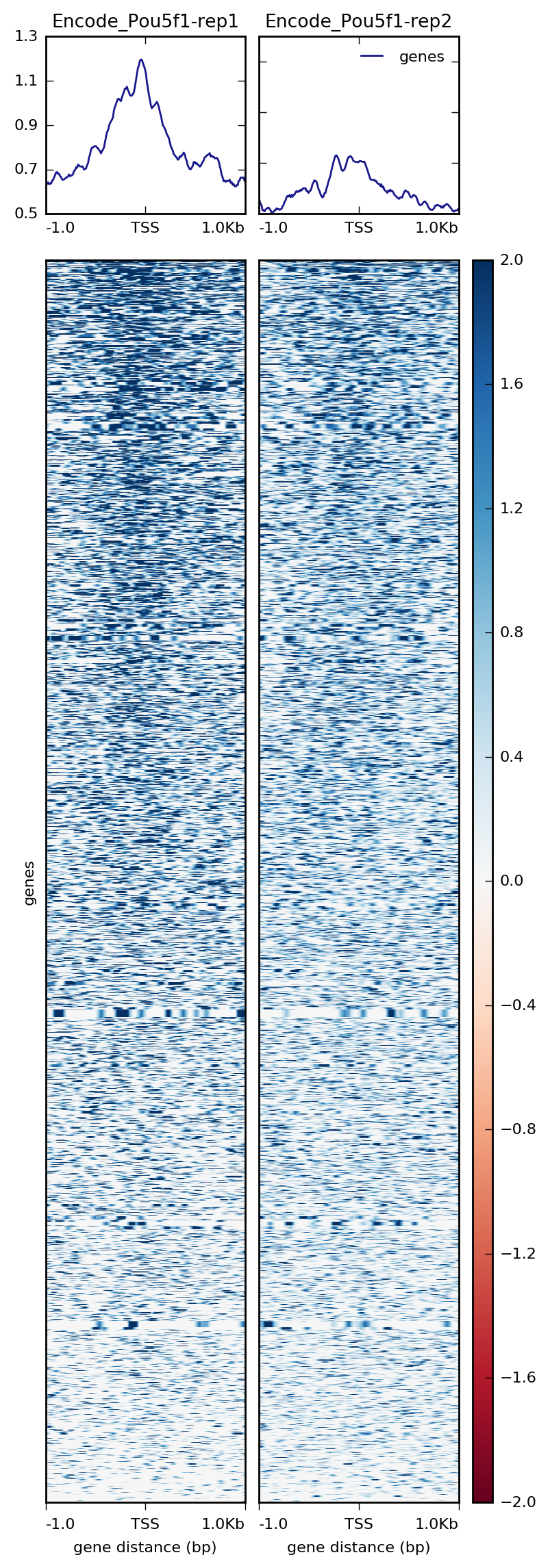
If we wanted both images in one single plot, we can do that with plotHeatmap and just removing the --whatToShow parameter.
$ plotHeatmap -m visualization/matrixPou5f1_TSS_chr12.gz \
-out visualization/figures/TSS_Pou5f1_profile-heatmap.png \
--colorMap RdBu \
--zMin -2 --zMax 2
NOTE: Both
plotProfileandplotHeatmaphave many options, including the ability to change the type of lines plotted and to plot by group rather than sample. We encourage you to explore the documentation to find out more detail.
Visualizing enrichment in differentially enriched regions
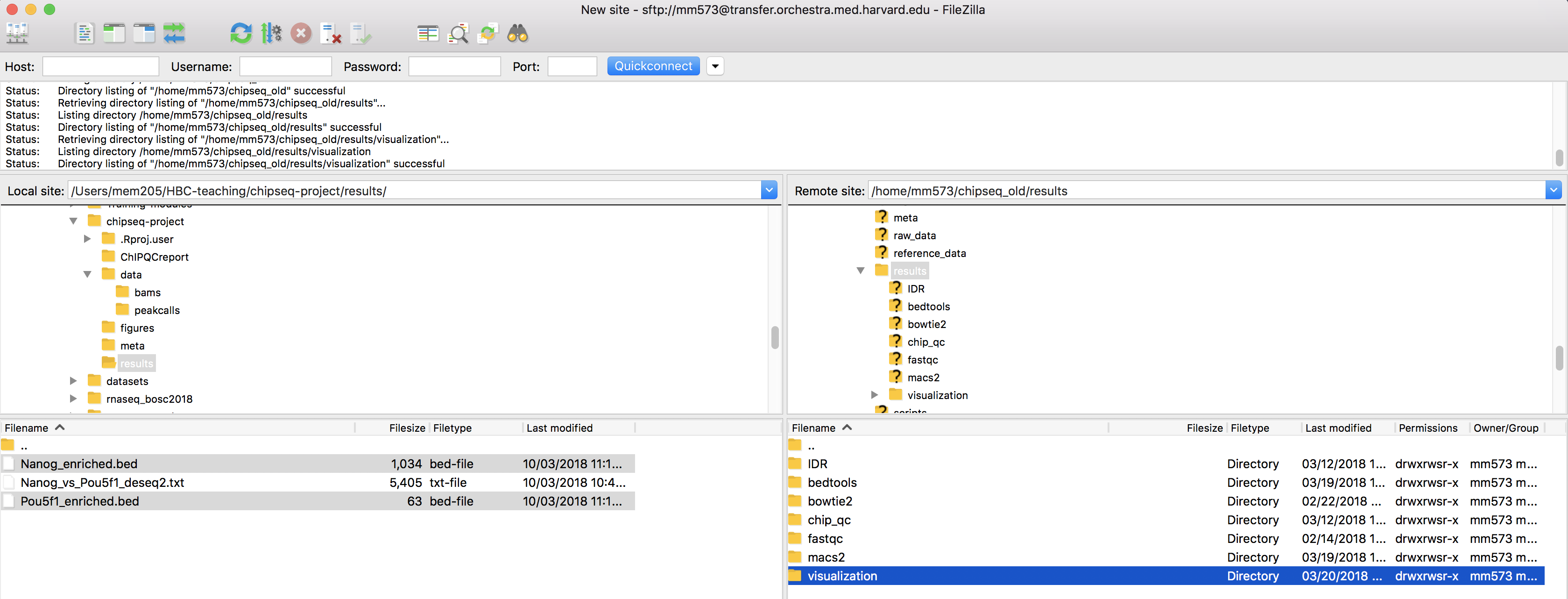
Previously, we had evaluated differential enrichment between the two factors in our study. We had found almost all of the peaks that were identfied were specific to Nanog and only one region that had significantly higher enrichment in Pou5f1. We can use the BED files we generated with DiffBind as input to deepTools and visualize enrichment in those regions to evaluate the differences in read density.
- Open up
FileZillaand copy over the BED files to O2 in~/chipseq/results/visualization:
Now we can use some of the deepTools commands we had explored previously. Note that we have changed the command from reference-point to scale-regions. In the scale-regions mode, all regions in the BED file are stretched or shrunken to the length in bases indicated by the user (--regionBodyLength).
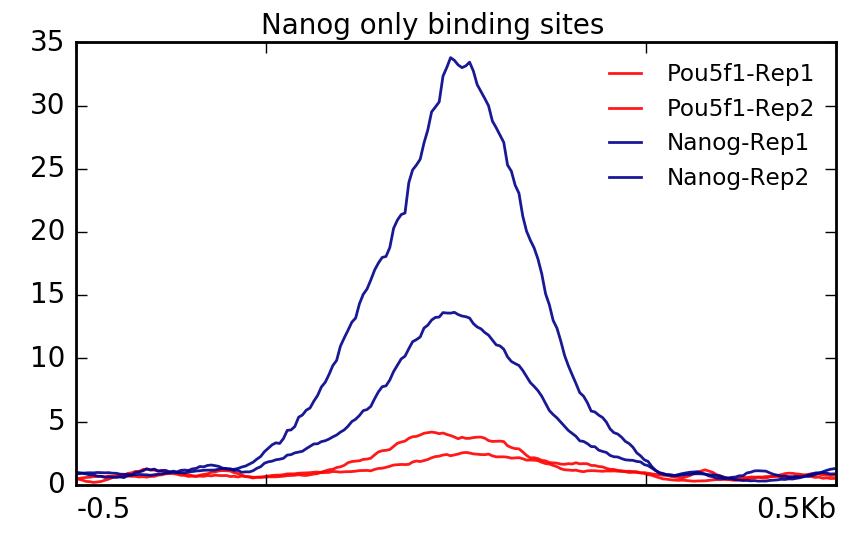
Let’s start with Nanog file which contains 33 regions that were identified as increased in enrichment compared to Pou5f1. The plot confirms what we had expected, that is, Pou5f1 don’t have much read depth in these regions.
$ computeMatrix scale-regions \
-R ~/chipseq/results/visualization/Nanog_enriched.bed \
-S /n/groups/hbctraining/chip-seq/full-dataset/bigWig/Encode_Pou5f1*.bw /n/groups/hbctraining/chip-seq/full-dataset/bigWig/Encode_Nanog*.bw \
--skipZeros \
-p 6 \
--regionBodyLength 2000 \
-a 500 -b 500 \
-o ~/chipseq/results/visualization/matrixAll_Nanog_binding_sites.gz
$ plotProfile -m visualization/matrixAll_Nanog_binding_sites.gz \
-out visualization/figures/Allsamples_NanogSites_profile.png \
--perGroup --plotTitle "" \
--samplesLabel "Pou5f1-Rep1" "Pou5f1-Rep2" "Nanog-Rep1" "Nanog-Rep2" \
-T "Nanog only binding sites" -z "" \
--startLabel "" \
--endLabel "" \
--colors red red darkblue darkblue
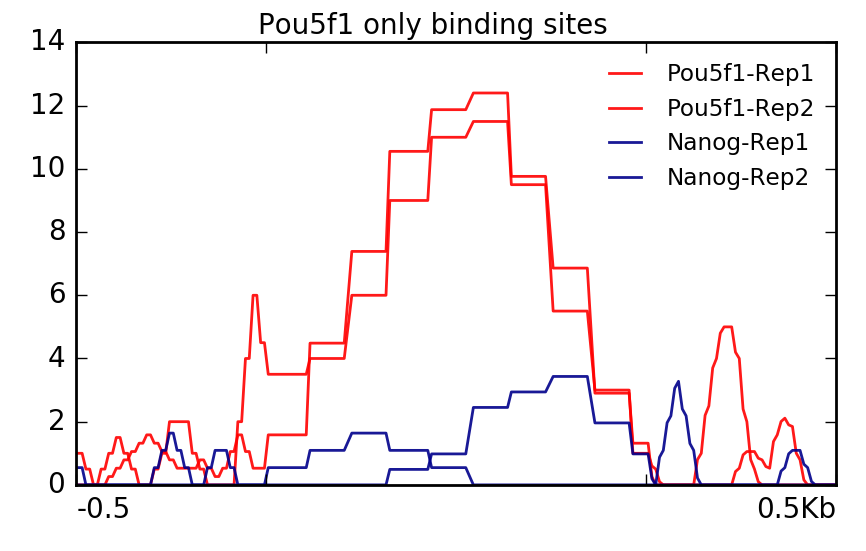
With Pou5f1, remember we only had one region. We are still able to plot this data but you will notice that it is a bit more boxy in nature. This is because values are not being averaged over multiple regions.
$ computeMatrix scale-regions \
-R ~/chipseq/results/visualization/Pou5f1_enriched.bed \
-S /n/groups/hbctraining/chip-seq/full-dataset/bigWig/Encode_Pou5f1*.bw /n/groups/hbctraining/chip-seq/full-dataset/bigWig/Encode_Nanog*.bw \
--skipZeros \
-p 6 \
--regionBodyLength 2000 \
-a 500 -b 500 \
-o ~/chipseq/results/visualization/matrixAll_Pou5f1_binding_sites.gz
$ plotProfile -m visualization/matrixAll_Pou5f1_binding_sites.gz \
-out visualization/figures/Allsamples_Pou5f1Sites_profile.png \
--perGroup --plotTitle "" \
--samplesLabel "Pou5f1-Rep1" "Pou5f1-Rep2" "Nanog-Rep1" "Nanog-Rep2" \
-T "Pou5f1 only binding sites" -z "" \
--startLabel "" --endLabel "" \
--colors red red darkblue darkblue
This lesson has been developed by members of the teaching team at the Harvard Chan Bioinformatics Core (HBC). These are open access materials distributed under the terms of the Creative Commons Attribution license (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.