Learning Objectives
- Describe current approaches for evaluating differences in cell proportions between groups
- Distinguish between cluster-based and cluster-free methods for DA analysis
- Run miloR for differential abundance analysis on VSM cells
Differential abundance of celltypes
Differential abundance (DA) analysis is a method used to identify celltypes with statistically significant changes in abundance between different biological conditions. The overall aim is to find sub-populations of cells in which the ratio of cells from the two conditions is significantly different from the ratios observed in the overall data. Methods for differential abundance have been successfully used in practice in both clinical and experimental settings. For example, these approaches highlighted an increased presence of granulocytes, monocytes, and B cells in fatal cases of COVID-19 (1).
The figure below is taken from a 2024 benchmarking study of DA approaches, and nicely illustrates DA effects.
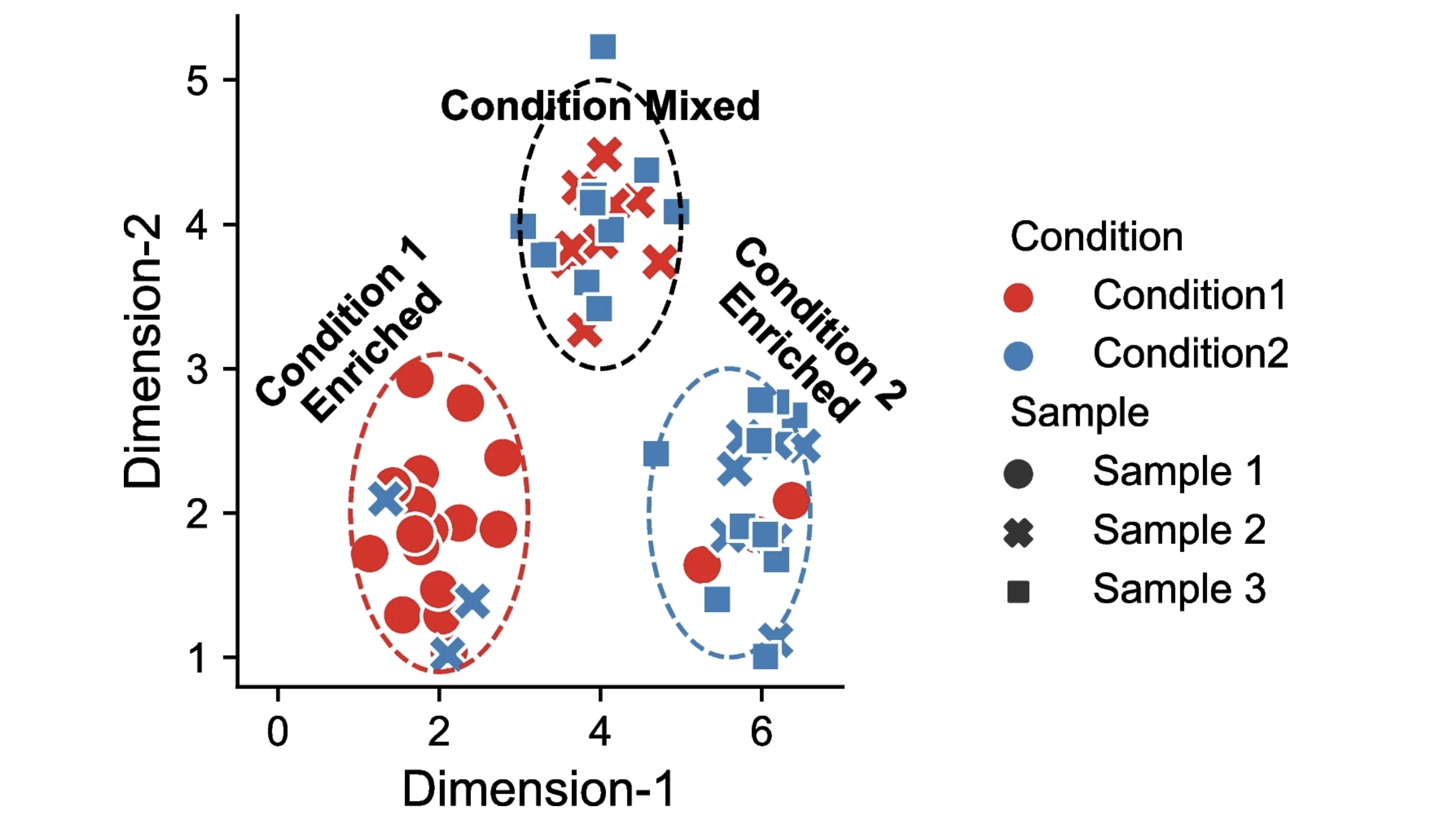
Cluster-based approaches for DA
These methods are dependent on having cells grouped into phenotypically similar cell populations, most classically aligning with specific cell types. Many single cell RNA-seq data analysis workflows produce a result with annotated sub-populations, making these tools very easy to implement as a next step.
The propellor method is a function that is part of the speckle R package, which uses cell level annotation information to calculate differential abundance estimates. First, cell type proportions are calculated for each sample. This results in matrix of proportions where the rows are the cell types and the columns are the samples. The matrix is then transformed such that a linear modeling framework can be applied. If there are exactly two groups, moderated t-tests are implemented; if there are more than two groups, the option is a moderated ANOVA test. These tests are moderated using an empirical Bayes framework, borrowing information across all cells, and finally false discovery rates are calculated.
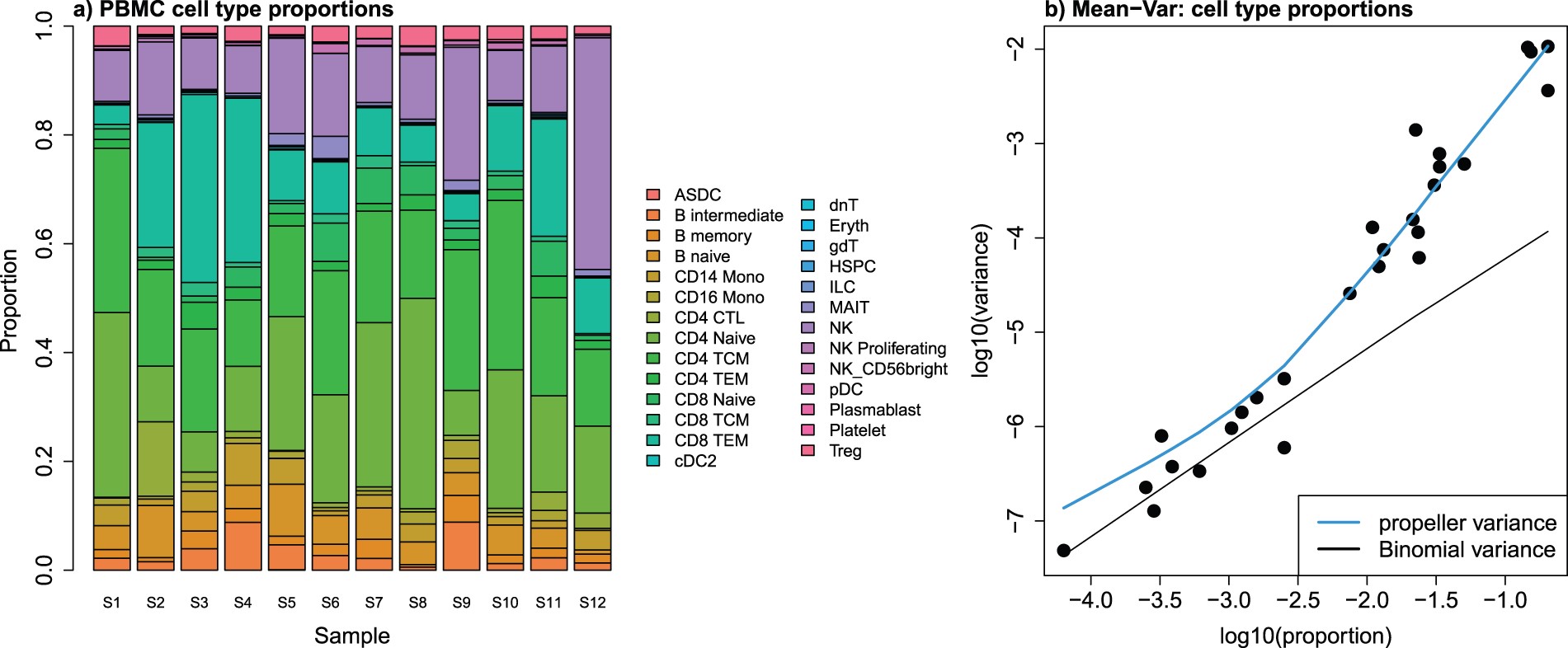
Image source: Phipson B. et al, 2022
While propellor and other approaches based on linear regression (i.e., scDC, diffcyt) transform the data to model data compositionality, they do not model the data count distribution. Modeling single-cell compositional data as counts is important as small datasets and rare cell types are characterized by a high noise-to-signal ratio, and modeling counts enables the down-weighting of small cell-group proportions compared to larger ones (Mangiola s. et al, 2023). The sccomp package is a generalized method for differential composition and variability analyses based on sum-constrained independent Beta-binomial distributions. The sccomp core algorithm, data integration, and visualization are outlined in the figure below. Two important features of this method include outlier detection and differential variability analysis.
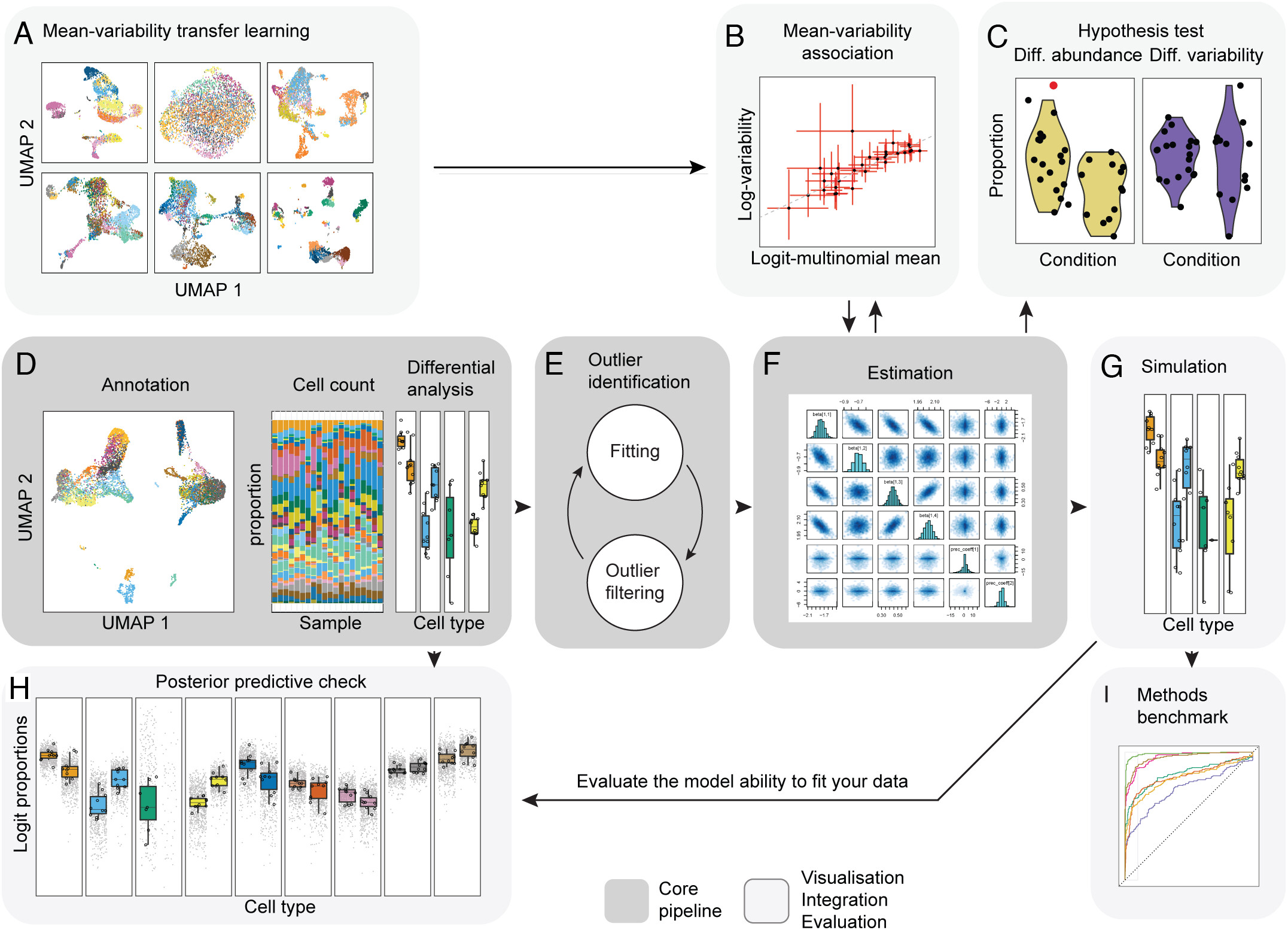
Cluster-free approaches
The clustering step can however be problematic, especially in cases where the sub-populations most responsive to the biological state do not fall into well-defined separate clusters.
- For example, subpopulations that are differentially abundant between conditions may be distributed among several adjacent clusters or, alternatively, encompass only a part of a cluster.
- A requirement for clustering could also compromise results for continuous processes where no clear cluster structure exists, such as cell cycles or certain developmental programs. For the above scenarios, differential abundance at a cluster level may miss the important molecular mechanisms that differentiate between the states.
In this lesson, we will explore the use of MiloR for differential abundance analysis. This tool does not rely on clustering of cells into discrete groups and instead makes use of k-nearest neighbor (KNN) graphs, a common data structure that is embedded in many single-cell analyses (Dann E. et al, 2021.)
NOTE: Although we present the code and workflow for MiloR in this lesson, this does not suggest this method to be a conclusive best practice for DA analysis.
Differential abundance analysis with MiloR
Looking at single-cell datasets on a cluster/celltype level is a very common mode of analysis. However, perhaps you have questions on the more subtle shifts within a certain cell population. The tool miloR allows you to look more deeply into smaller neighborhoods of cells by utilizing differential abundance testing on the k-nearest neighbor graph.
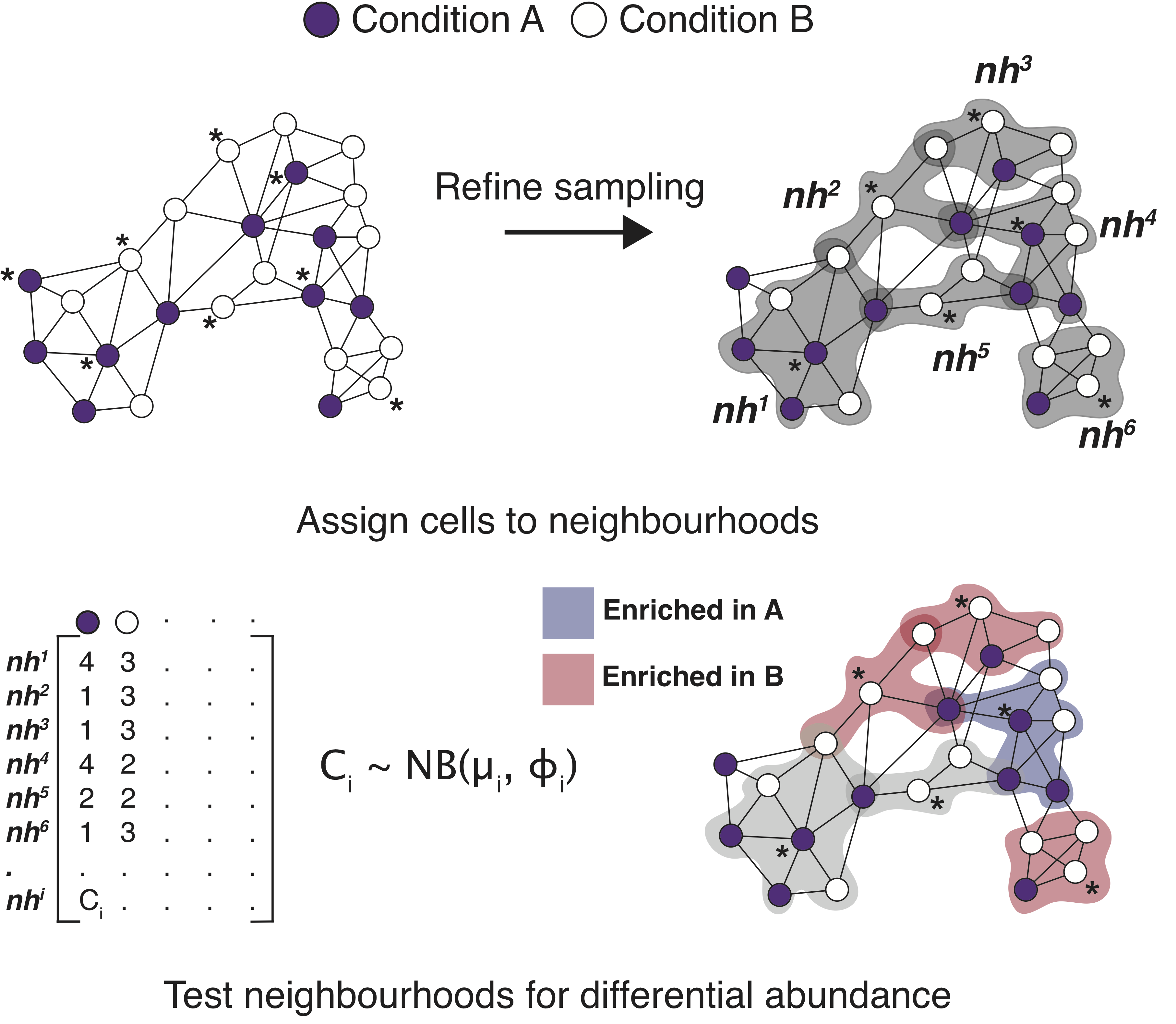
Image source: Dann E. et al, 2021
The general method of this tool is to assign cells to neighborhoods based upon a latent space (typically PCA) and neighborhood graph. Ultimately, we generate a neighborhood by counts matrix. These counts are modelled with a negative bionomial generalized linear model, which is then put through hypothesis testing to identify significantally differentially abundant neighborhoods with associated fold change values.
Create new script
To start, open a new Rscript file, and start with some comments to indicate what this file is going to contain:
# Single-cell RNA-seq analysis - Differential abundance analysis with MiloR
Save the Rscript as miloR_analysis_scrnaseq.R.
Load libraries
As usual, let us load the libraries needed at the beginning of our script.
library(Seurat)
library(tidyverse)
library(SingleCellExperiment)
library(dplyr)
library(miloR)
library(EnhancedVolcano)
Select cell subsets
For continuity, let us take a look at the differences between the TN and cold7 conditions. Here we are also going to set our seed so that we are all introducing the same randomness values in later steps.
set.seed(1234)
# Subset to condition of interest
# seurat <- readRDS("data/BAT_GSE160585_final.rds")
seurat_sub <- subset(seurat, subset = (condition %in% c("TN", "cold7")))
MiloR generates the neighborhoods based upon the UMAP coordinates supplied, so we will re-run the necessary steps from our Seurat pipeline on this new subset. Since we have fewer cells than the larger datset, will use 30 PCA dimensions calculated from 2,000 highly variable genes (HVG). Following a typical Seurat workflow, we then calculate UMAP coordinates, neighborhoods, and clusters for later comparisons. We are also supplying specific names for the graphs and cluster names to avoid overwriting the previous metadata.
# HVG, PCA, UMAP, neighborhoods, calculate clusters
seurat_sub <- FindVariableFeatures(seurat_sub, verbose=FALSE, nfeatures=2000)
seurat_sub <- ScaleData(seurat_sub, verbose=FALSE)
seurat_sub <- RunPCA(seurat_sub, verbose = FALSE)
seurat_sub <- RunUMAP(seurat_sub, dims = 1:30, verbose=FALSE)
seurat_sub <- FindNeighbors(seurat_sub, dims = 1:30,
graph.name="sub_graph", verbose=FALSE)
# Determine the clusters at resolution 0.4
seurat_sub <- FindClusters(seurat_sub, cluster.name="sub_clusters",
resolution=0.4, graph.name="sub_graph",
verbose=FALSE)
DimPlot(seurat_sub, group.by=c("condition", "sub_clusters", "celltype"))
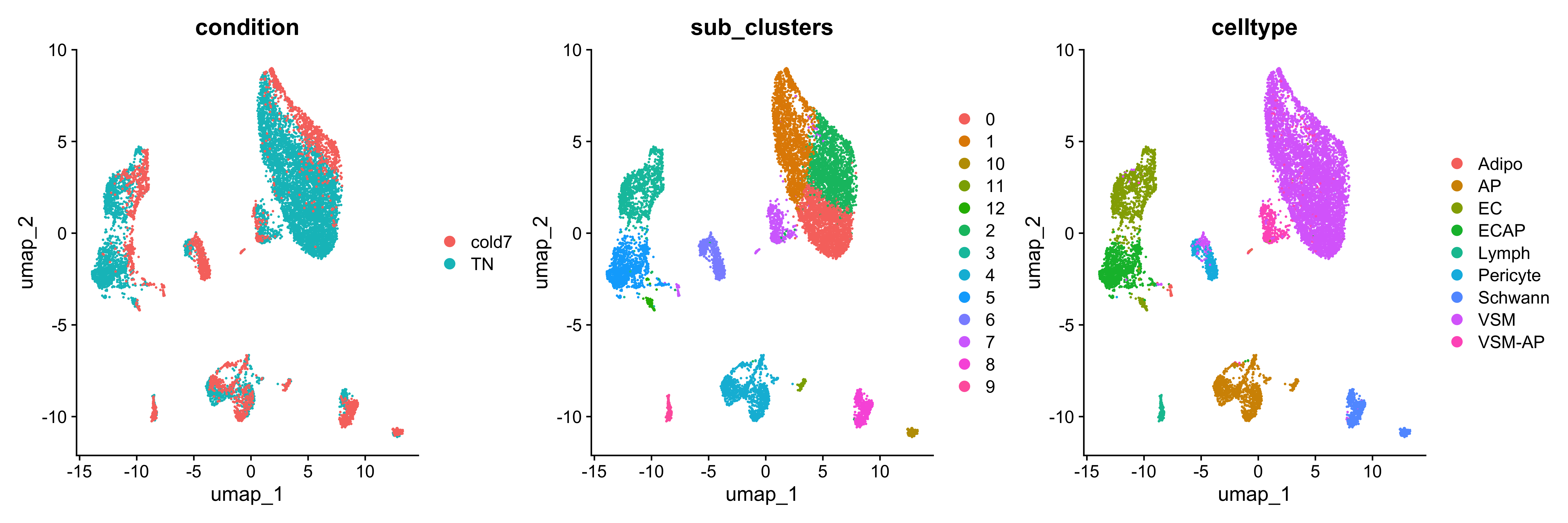
We see a distinct separation of cells based upon which sample the cells come from, which will allow us to clearly identify changes in our dataset by condition with MiloR.
Creating single cell experiment
In order to make use of the MiloR package, we must format our datset in the correct way. There is another data structure known as SingleCellExperiment that is commonly used to analyze single-cell experiments. We will first convert our Seurat object and investigate the underlying structure so that we can easily use and modify the object according to our needs.
# Create SingleCellExperiment object
DefaultAssay(seurat_sub) <- "RNA"
sce <- as.SingleCellExperiment(seurat_sub)
A SingleCellExperiment stores metadata, counts matrix, and reductions in the following way:
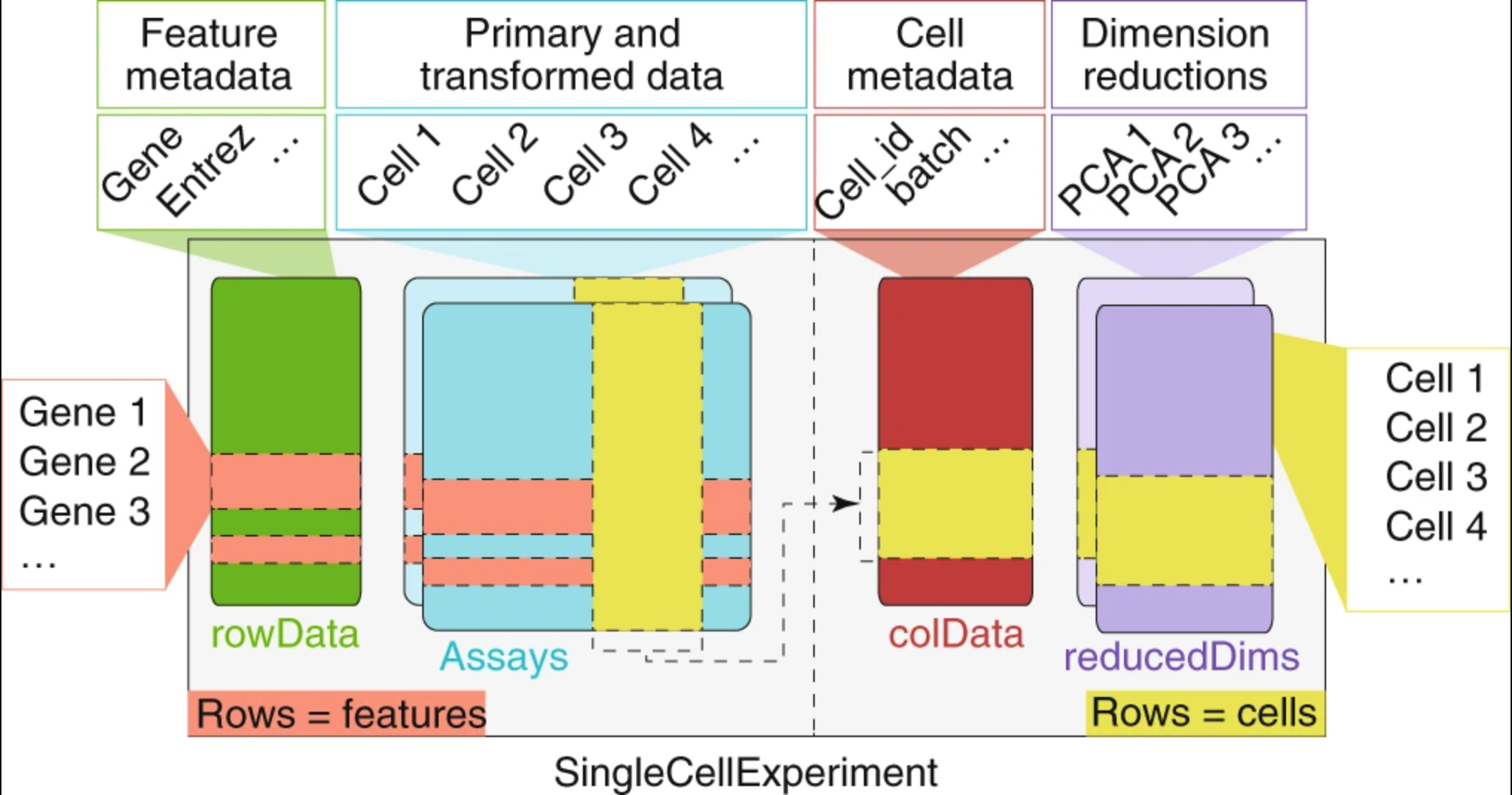
Image credit: Amezquita, R.A., Lun, A.T.L., Becht, E. et al, 2019
We can use the functions from the SingleCellExperiment package to extract the different components. Let’s explore the counts and metadata for the experimental data.
## Explore the raw counts for the dataset
# Check the assays present
assays(sce)
# Explore the raw counts for the dataset
dim(counts(sce))
# Access the first 6 genes and cells in the counts matrix
counts(sce)[1:6, 1:6]
6 x 6 sparse Matrix of class "dgCMatrix"
AAACCCACAGCTATTG_1 AAACCCAGTCGGTAAG_1 AAACCCAGTTCCGGTG_1 AAACGAAAGGGCGAAG_1 AAACGAACATTCGATG_1 AAACGAAGTAGCTCGC_1
Xkr4 . . . . . .
Gm1992 . . . . . .
Rp1 . . . . . .
Sox17 . 1 . . . .
Mrpl15 2 1 . 2 . 1
Lypla1 . . . . . .
We see the raw counts data is a cell by gene sparse matrix with the same genes (rows) and columns (cells) as in our Seurat object.
Next, we can get an idea of how to access the metadata in our SCE object by using the colData() function:
# Explore the cellular metadata for the dataset
dim(colData(sce))
head(colData(sce))
Creating Milo object
Now that we better understand how to use a SingleCellExperiment, we can convert it to a Milo object. While there are slight differences in this object, the basic idea of how to access metadata and counts information is consistent with a SingleCellExperiment. To avoid re-computing PCA and UMAP coordinates, we are going to store the Seurat generated values in the Embeddings slot of our Milo object.
# Create miloR object
milo <- Milo(sce)
# Store previously computed PCA and UMAP values
reducedDim(milo, "PCA") <- Embeddings(seurat_sub, reduction="pca")
reducedDim(milo, "UMAP") <- Embeddings(seurat_sub, reduction="umap")
milo
class: Milo
dim: 19771 11148
metadata(0):
assays(2): counts logcounts
rownames(19771): Xkr4 Gm1992 ... CAAA01118383.1 CAAA01147332.1
rowData names(0):
colnames(11148): AAACCCACAGCTATTG_1 AAACCCAGTCGGTAAG_1 ... TTTGACTAGGCTTCCG_16 TTTGTTGAGGGACAGG_16
colData names(18): orig.ident nCount_RNA ... sub_clusters ident
reducedDimNames(2): PCA UMAP
mainExpName: RNA
altExpNames(0):
nhoods dimensions(2): 1 1
nhoodCounts dimensions(2): 1 1
nhoodDistances dimension(1): 0
graph names(0):
nhoodIndex names(1): 0
nhoodExpression dimension(2): 1 1
nhoodReducedDim names(0):
nhoodGraph names(0):
nhoodAdjacency dimension(2): 1 1
The major differences between Milo and a SingleCellExperiment comes from the slots where nhood values are stored, as this is information that is uniquely stored in our Milo object. These values will be automatically populated as we use the various function built into the package.
Milo workflow
Now that we have our dataset in the correct format, we can begin utilizing the Milo workflow.
Creating neighborhoods
Step one is to generate the k-nearest neighborhood graph with the buildGraph() function. The parameters include selecting k neighbors and d dimensions (PCs):
k: An integer scalar that specifies the number of nearest-neighbours to consider for the graph building. Default is 10.d: The number of dimensions to use if the input is a matrix of cells. Default is 50.
# Build the graph
traj_milo <- buildGraph(milo, k = 10, d = 30)
Afterwards we use the makeNhoods() function to define the neighborhoods based upon the graph calculated before. These neighborhoods are then refined further by evaluating the median PC values and vetrices to generate a minimal, but informative graph of the data. The values assigned to the parameters for this function should be consistent with the ones that were chosen when the graph was built in the previous step:
prop: A double scalar that defines what proportion of graph vertices to randomly sample. Must be 0 <prop< 1. Default is 0.1.k: An integer scalar - the same k used to construct the input graph. Default is 21.d: The number of dimensions to use if the input is a matrix of cells X reduced dimensions. Default is 30.
Once we generate these neighborhoods, we can visualize the number of cells that belong to each neighborhood as a histogram. If the number of cells in each neighborhood are too small for our given dataset, this could be an indication that we need to select a different value for k.
traj_milo <- makeNhoods(traj_milo, prop = 0.1, k = 10, d = 30, refined = TRUE)
plotNhoodSizeHist(traj_milo)
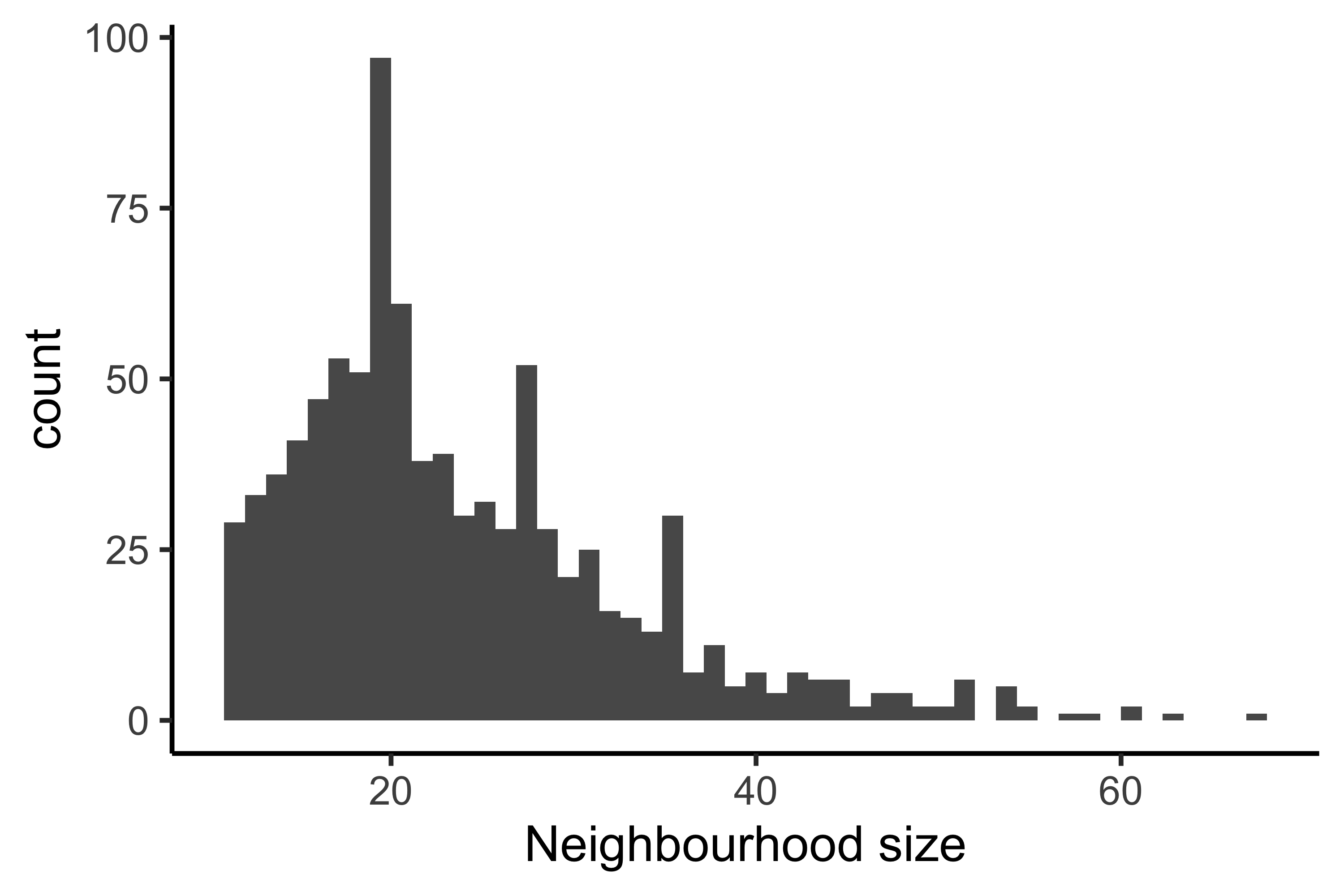
Creating metadata
Now that we have identified which cells belong to which neighborhoods, we can quantify how many cells from each sample belong to each neighborhood.
# Count number of cells per neighborhood
traj_milo <- countCells(traj_milo,
meta.data = colData(traj_milo),
sample="sample")
nhoodCounts(traj_milo) %>% head()
6 x 8 sparse Matrix of class "dgCMatrix"
Sample_1 Sample_2 Sample_9 Sample_10 Sample_7 Sample_8 Sample_15 Sample_16
1 8 6 . 4 . . . .
2 6 . 9 11 . . . .
3 . 2 17 10 . . . .
4 . 1 . . 10 11 . 1
5 6 2 10 29 1 . . .
6 1 3 2 9 . 1 . 2
With this sample-level information, we can account for technical variability across replicates. To define which samples belong to which condition, we next create a metadata dataframe. This table will contain all of the relevant pieces of information for the comparisons we want to run, including the sample names. In the case of this experiment, we need the columns sample and condition.
# Create metadata
# Subset to columns of importance
# Get distinct/unique values
# Set sample as the rownames
traj_design <- colData(traj_milo) %>%
data.frame() %>%
select(sample, condition) %>%
distinct() %>%
remove_rownames() %>%
column_to_rownames("sample")
# Reorder rownames to match columns of nhoodCounts()
order_rows <- colnames(nhoodCounts(traj_milo))
traj_design <- traj_design[order_rows, , drop=FALSE]
traj_design
condition
Sample_1 TN
Sample_2 TN
Sample_9 TN
Sample_10 TN
Sample_7 cold7
Sample_8 cold7
Sample_15 cold7
Sample_16 cold7
Now we have all the relevant information to begin testing differential abundance!
Run differential abundance
To test the differences in neighborhoods, we first calculate the Euclidean distances between nearest neighborhoods using the PCs that the graph was first constructed on with the calcNhoods() function. With the distances computed for each neighborhood in our dataset, we can begin assessing the overlap in neighborhoods. This is accomplished with the Spatial FDR correction where each hypothesis test p-values are adjusted based upon the nearest neighbor distances.
This step may take some time to run for a large dataset.
# Calculate differential abundance
# This may take some time to run
traj_milo <- calcNhoodDistance(traj_milo, d=30)
Now we can finally calculate the differential abundance across the neighborhoods with testNhoods(). We specify the design, or the model we want to use in the comparison. The columns used in design must be found within the design.df metadata dataframe. This results in a dataframe with the following columns:
logFC: Numeric, the log fold change between conditions or, for an ordered/continous variable, the per-unit change in (normalized) cell counts per unit-change in experimental variable.logCPM: Numeric, the log counts per million (CPM), which equates to the average log normalized cell counts across all samples.F: Numeric, the F-test statistic from the quali-likelihood F-test implemented in edgeR.PValue: Numeric, the unadjusted p-value from the quasi-likelihood F-test.FDR: Numeric, the Benjamini & Hochberg false discovery weight computed from p.adjust.Nhood: Numeric, a unique identifier corresponding to the specific graph neighbourhood.SpatialFDR: Numeric, the weighted FDR, computed to adjust for spatial graph overlaps between neighbourhoods.
da_results <- testNhoods(traj_milo,
design = ~ condition,
design.df = traj_design)
da_results %>% head()
logFC logCPM F PValue FDR Nhood SpatialFDR condition condition_fraction
1 5.077949 10.92841 8.328449 0.003918351 0.010144926 1 0.008677397 TN 1.0000000
2 5.595769 11.22084 9.946825 0.001620099 0.006318517 2 0.005170829 TN 1.0000000
3 6.026816 11.49294 10.713690 0.001070141 0.006318517 3 0.005170829 TN 1.0000000
4 -3.946278 11.23250 6.741285 0.009446174 0.019465082 4 0.016947029 cold7 0.9565217
5 4.703667 11.82490 7.968536 0.004777138 0.011760113 5 0.010144964 TN 0.9791667
6 1.874743 11.02911 1.997329 0.157634216 0.197811182 6 0.181707364 TN 0.8333333
Now that we have our neighborhoods, we can add extra metadata to these results. For example, we can annotate these groups by the percentage of cells in the neighborhood that belong to each condition using the annotateNhoods() function. Bear in mind that the coldata_col variable must be a column found in colData() of the milo object. This will create two new columns where condition represents what condition the majority of cells belong to, while condition_fraction represent the percent of cells annotated with that condition.
The developers of MiloR were cognicent of the fact that there may be neighborhoods of cells where there is a mix of two conditions. In their vignette, they recommend categorizing these neighborhoods as “Mixed”.
# Annotate neighborhoods by condition
da_results <- annotateNhoods(traj_milo, da_results, coldata_col = "condition")
# Categorize neighborhoods with < 70% of one condition as mixed
da_results$anno_condition <- ifelse(da_results$condition_fraction < 0.7,
"Mixed",
da_results$condition)
# Annotate neighborhoods by celltype
da_results <- annotateNhoods(traj_milo, da_results, coldata_col = "celltype")
# Categorize neighborhoods with < 70% of one celltype as mixed
da_results$anno_celltype <- ifelse(da_results$celltype_fraction < 0.7,
"Mixed",
da_results$celltype)
# Annotate neighborhoods by cluster
da_results <- annotateNhoods(traj_milo, da_results, coldata_col = "sub_clusters")
The final piece of information is added to this dataframe of differential abundance results with the groupNhoods() function. This will run the louvain clustering algorithm to identify neighborhoods that are overlapping and similar to one another. This max.lfc.delta specifies a cutoff of fold change difference where two neighborhoods would not be considered a part of the same group. We are setting a value of 5, which is quite high in order to minimize the number of neighborhood groups (similar to the resolution we set for louvain clustering).
Note that the exact number of groups may vary due to randomness in the model. Even if the results are not identical to what is displayed here, the general trends of the data should be similar.
# Find group structure of neighborhoods
da_results <- groupNhoods(traj_milo, da_results, max.lfc.delta = 5)
da_results %>% head()
logFC logCPM F PValue FDR Nhood SpatialFDR condition condition_fraction anno_condition sub_clusters sub_clusters_fraction celltype celltype_fraction anno_celltype NhoodGroup
1 5.077949 10.92841 8.328449 0.003918351 0.010144926 1 0.008677397 TN 1.0000000 TN 3 1.0000000 EC 1.0000000 EC 1
2 5.595769 11.22084 9.946825 0.001620099 0.006318517 2 0.005170829 TN 1.0000000 TN 2 0.8076923 VSM 0.9615385 VSM 2
3 6.026816 11.49294 10.713690 0.001070141 0.006318517 3 0.005170829 TN 1.0000000 TN 5 1.0000000 ECAP 1.0000000 ECAP 3
4 -3.946278 11.23250 6.741285 0.009446174 0.019465082 4 0.016947029 cold7 0.9565217 cold7 1 1.0000000 VSM 1.0000000 VSM 4
5 4.703667 11.82490 7.968536 0.004777138 0.011760113 5 0.010144964 TN 0.9791667 TN 1 1.0000000 VSM 0.9791667 VSM 5
6 1.874743 11.02911 1.997329 0.157634216 0.197811182 6 0.181707364 TN 0.8333333 TN 0 0.8333333 VSM 1.0000000 VSM 6
Visualization
Now, with all the information we have, we can visualize the differential abundance results using the UMAP coordinates that we initially supplied.
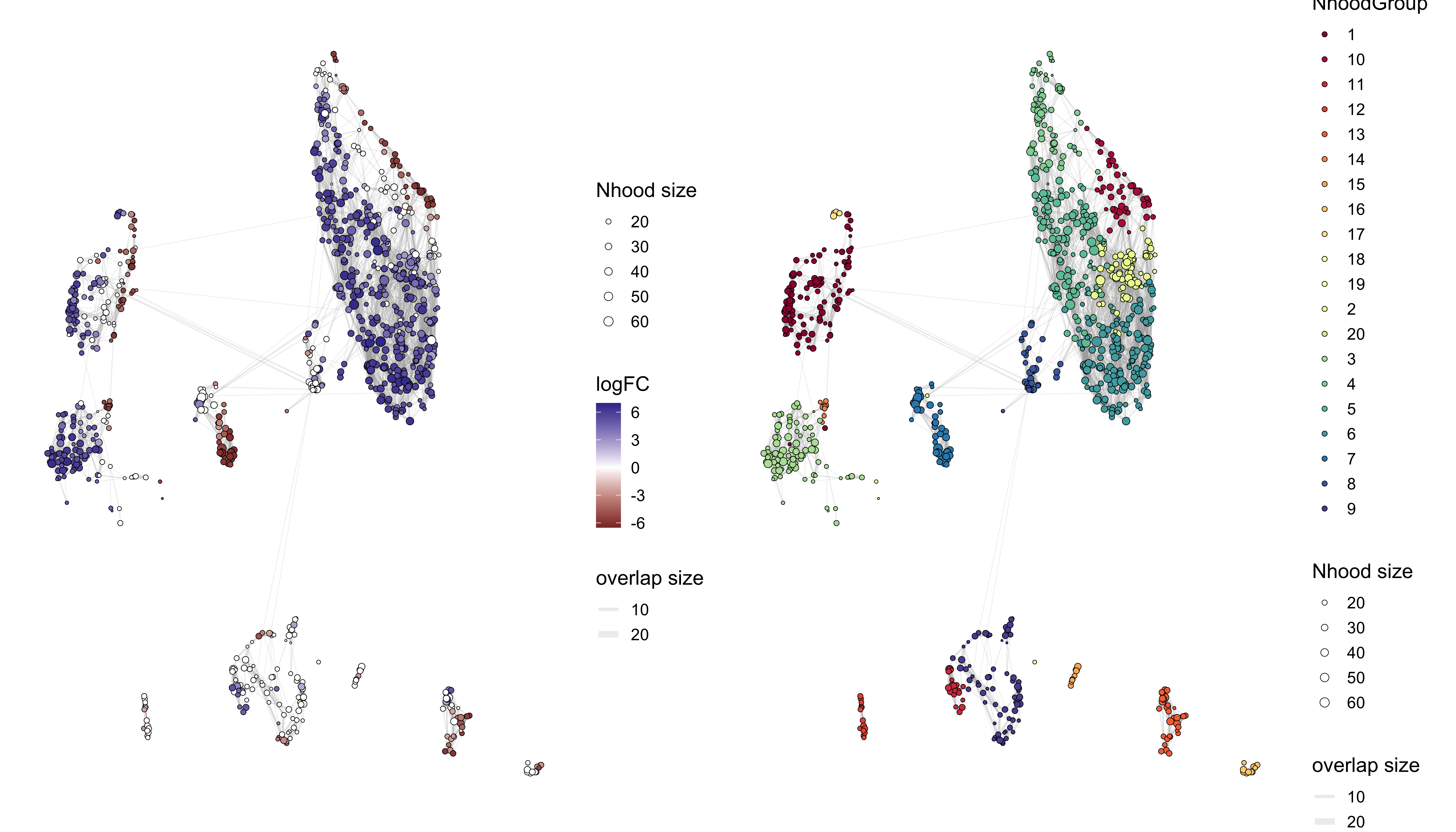
Neighborhoods overlaid UMAP
Each dot represents a different neighborhood, with the size of the dot representing how many cells belong to that neighborhood. The color of the circle represent the log-fold change for that neighborhood, from the da_results object. You may notices that there are many neighborhoods that do not contain any color, which is an indication that the FDR value did not meet that alpha value supplied.
We can also represent the neighborhoods according to the group structure that was calculated with the groupNhoods() function. The expectation here would be that the group structure bears a resemblance the clusters calculated after subsetting the data.
traj_milo <- buildNhoodGraph(traj_milo)
p1 <- plotNhoodGraphDA(traj_milo, da_results, alpha=0.1)
p2 <- plotNhoodGroups(traj_milo, da_results)
p1 + p2
Bee swarm plots
Another built in visualization can be accessed with the plotDAbeeswarm() function. In these visualizations, each point represents a neighborhood, which are grouped together according to the group.by parameter. Along the x-axis, these neighborhoods are spread out according to their log fold change score. This clearly shows the distribution of significant fold changes across different groups.
To begin, let’s look at the change in neighborhood expression across our two conditions.
plotDAbeeswarm(da_results, group.by = "anno_condition")
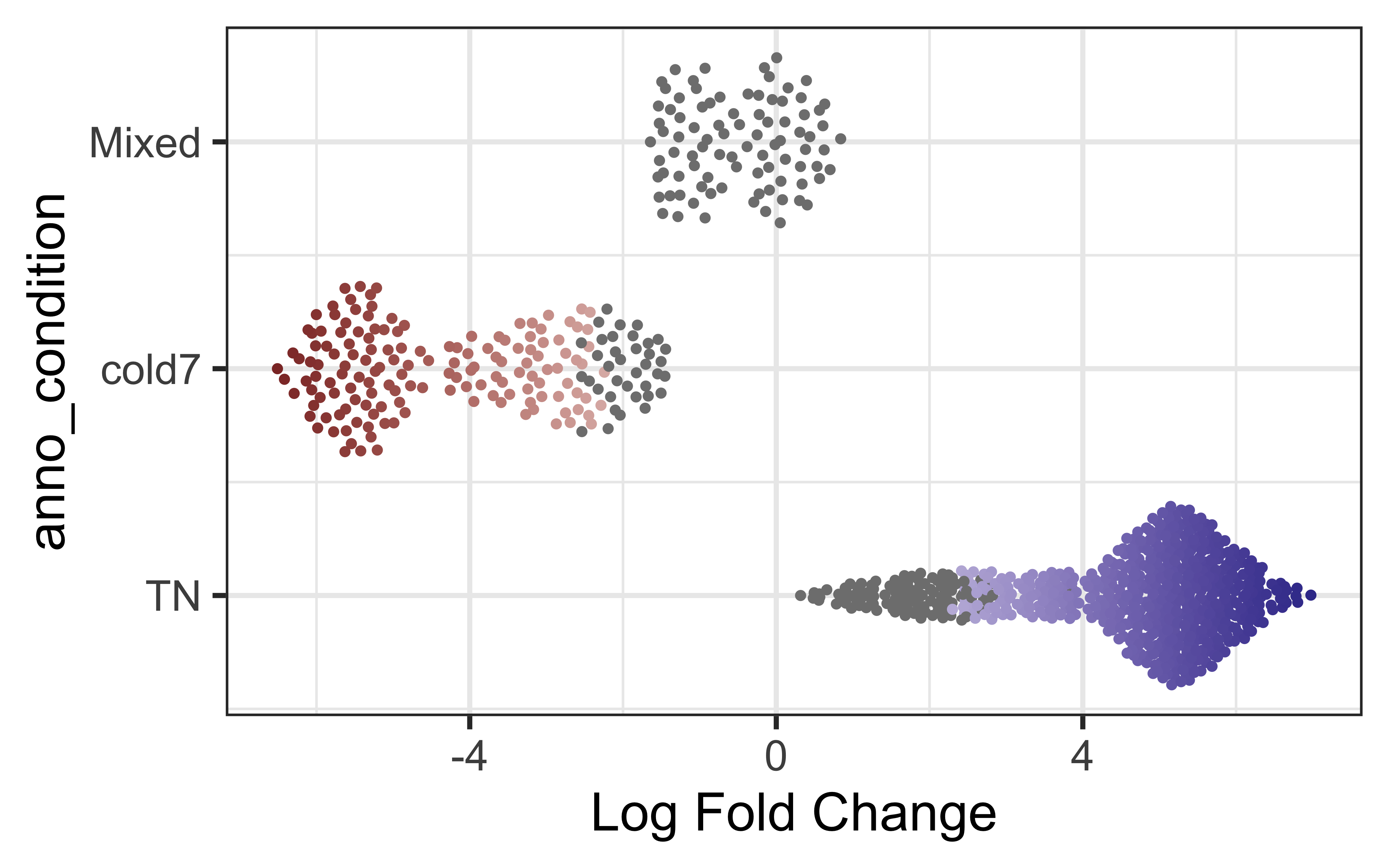
As expected, we see a clear FDR divide based upon condition as that was the design variable we computed the different neighborhoods on.
We can additionally take a look at the annotated celltypes to see how the neighborhoods distribute across the celltypes.
plotDAbeeswarm(da_results, group.by = "anno_celltype")
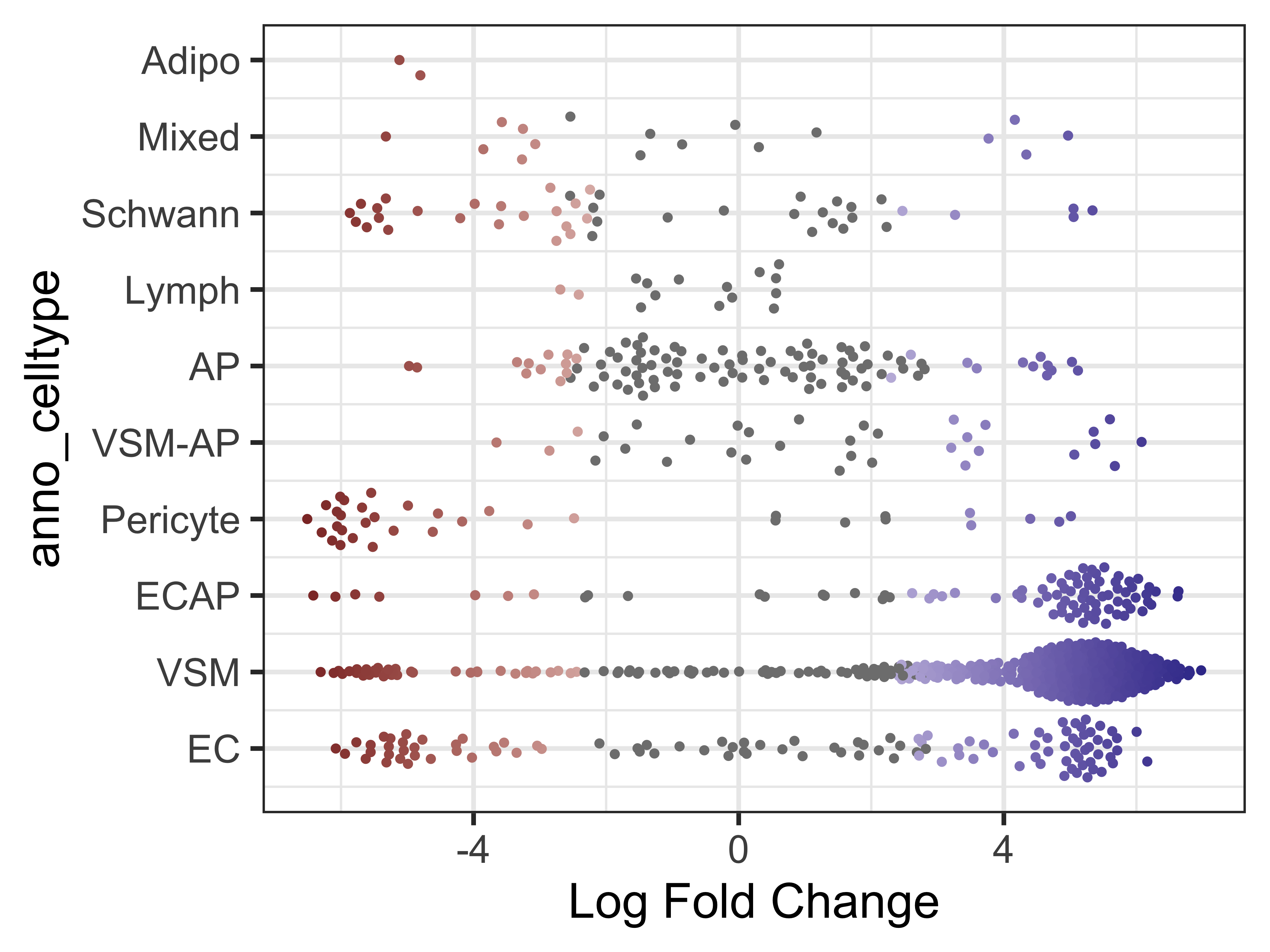
Note that in most celltypes, there appears to be a mix of both cold7 and TN neighborhoods (as indicated by the fold change values). This is an indication that these celltypes are shared across both conditions.
Finally, we can make the same visualization for the neighborhood groups. This will help us identify two different groups we may be interested in running a DGE analysis in the next step.
plotDAbeeswarm(da_results, group.by = "NhoodGroup")
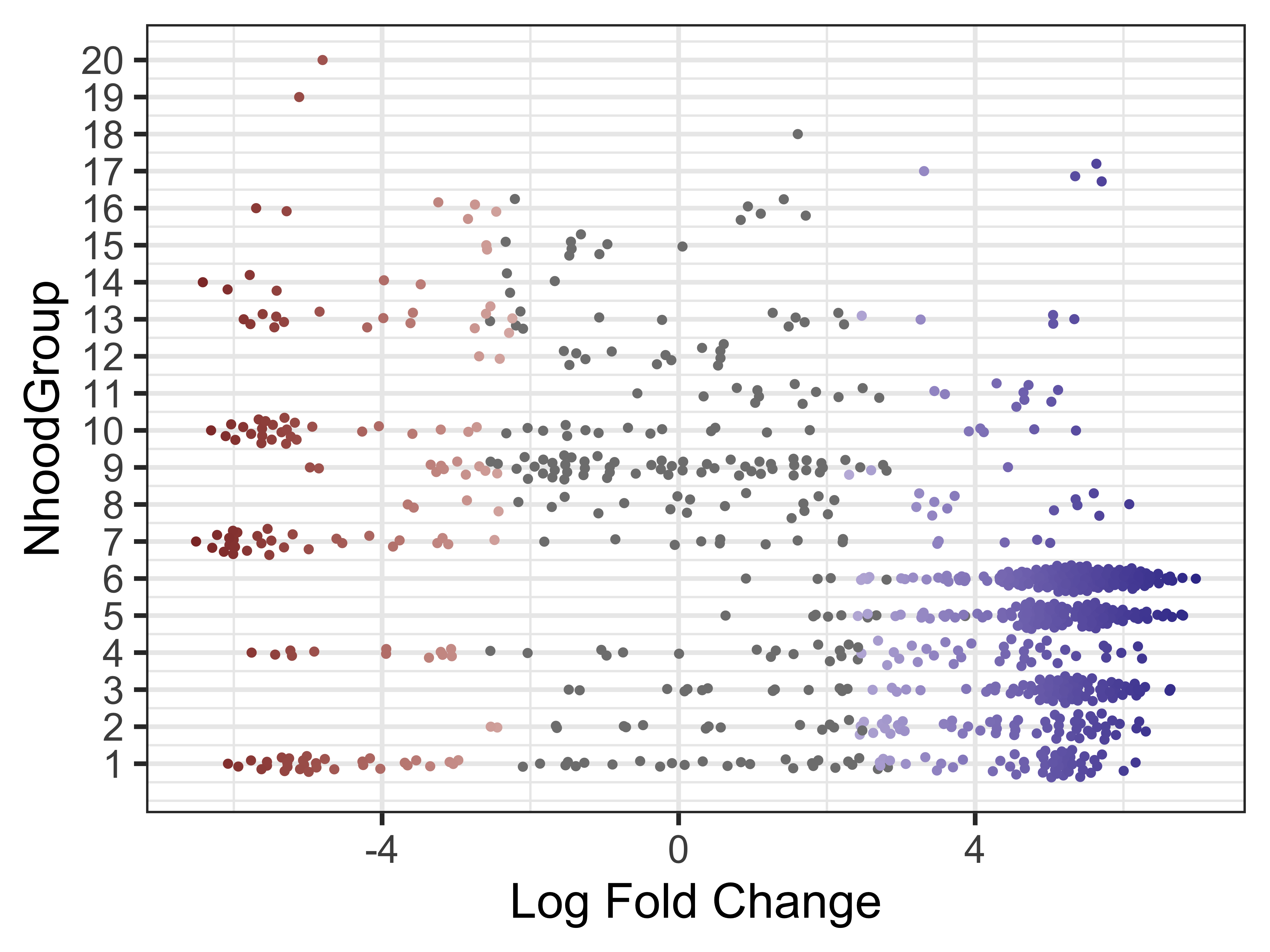
Neighborhood differential genes
Within the Milo package, we can even run a DGE analysis between different groups in our DA_results dataframe. We can test one group vs. rest of cells (similar to the FindAllMarkers() function within Seurat) with the findNhoodGroupMarkers() function. Ultimately this function makes use of the limma package. In doing so, we will have a LogFC_{group} and adj.P.Val_{group} for every single gene we specifiy. For simplicity, we will use the 2,000 highly variable genes. Additionally, to take into account variability across replicates, we set the aggregate.samples parameter to be true and specify which metadata column (in colData) indicates which cell comes from which sample.
# Use variable genes
hvgs <- VariableFeatures(seurat_sub)
nhood_markers <- findNhoodGroupMarkers(traj_milo,
da_results,
subset.row = hvgs,
aggregate.samples = TRUE,
sample_col = "sample")
If there are two neighborhoods groups of interest, instead we can run DE on just those two subsets with the subset.nhoods argument. As an example, let us look at neighborhood groups 6 and 7.
You may have different groups due to the randomness that is introduced during neighborhood grouping. Pick two groups that appear quite different in your bee swarm plots.
# Compare group 6 and 7
nhood_markers <- findNhoodGroupMarkers(traj_milo,
da_results,
subset.row = hvgs,
subset.nhoods = da_results$NhoodGroup %in% c('6', '7'),
aggregate.samples = TRUE, sample_col = "sample")
nhood_markers %>% arrange(abs(logFC_6)) %>% tail()
GeneID logFC_6 adj.P.Val_6 logFC_7 adj.P.Val_7
1995 Art3 -2.607195 2.897275e-12 2.607195 2.897275e-12
1996 Mgp -2.715875 1.439939e-07 2.715875 1.439939e-07
1997 Kcnj8 -2.824452 1.312572e-10 2.824452 1.312572e-10
1998 Meg3 -3.018706 4.580461e-13 3.018706 4.580461e-13
1999 Myh11 3.414083 2.113784e-13 -3.414083 2.113784e-13
2000 Rgs5 -4.097856 4.053591e-15 4.097856 4.053591e-15
Same as we have been doing throughout the workshop, we can then create a volcano plot of the DGE results.
EnhancedVolcano(nhood_markers,
nhood_markers$GeneID,
x = "logFC_6",
y = "adj.P.Val_6",
FCcutoff = 0.5,
pCutoff = 0.01,
title="Neighborhood group 6 vs 7")
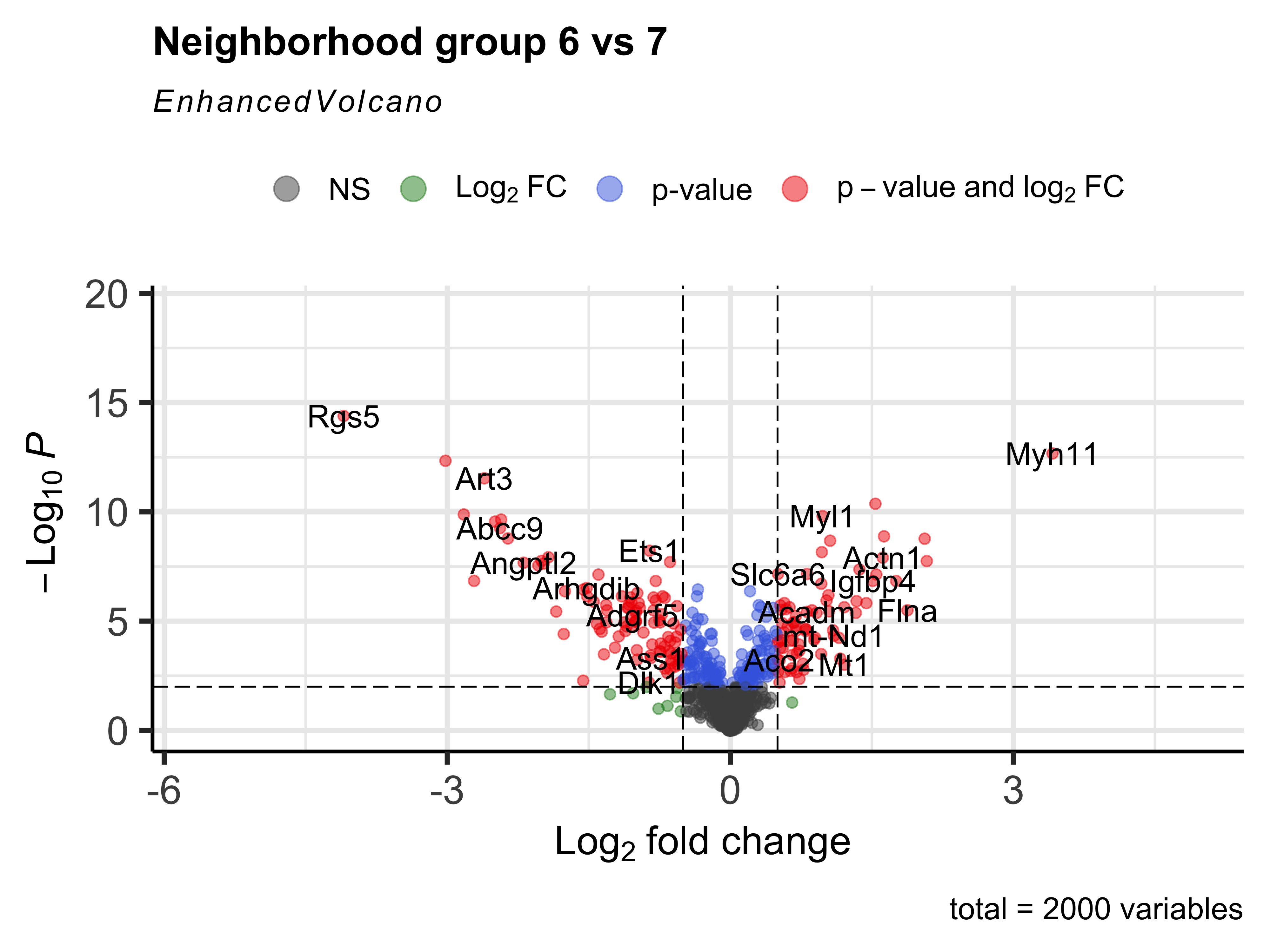
We can even generate a heatmap of expression per-neighborhood with the plotNhoodExpressionGroups() function. First, we can identify the top 10 genes in neighborhood group 6 based upon the log-fold change value. We can then supply those genes into our function and again subset the groups to 6 and 7.
This visualization represents the averaged expression of each gene among the cells in each each neighborhood.
# Make sure marker gene is in dataset
# P-value < 0.01
# Select LFC > 1
# Sort genes by LFC score
markers <- nhood_markers %>%
subset(GeneID %in% rownames(traj_milo)) %>%
subset(adj.P.Val_6 < 0.01) %>%
subset(logFC_6 > 0.5) %>%
arrange(logFC_6)
# Top markers
genes <- markers$GeneID[1:10]
genes <- genes[!is.na(genes)]
# Heatmap of top marker genes for groups 6 and 7
plotNhoodExpressionGroups(traj_milo,
da_results,
features=genes,
subset.nhoods = da_results$NhoodGroup %in% c('6','7'),
scale=TRUE,
grid.space = "fixed",
cluster_features = TRUE)
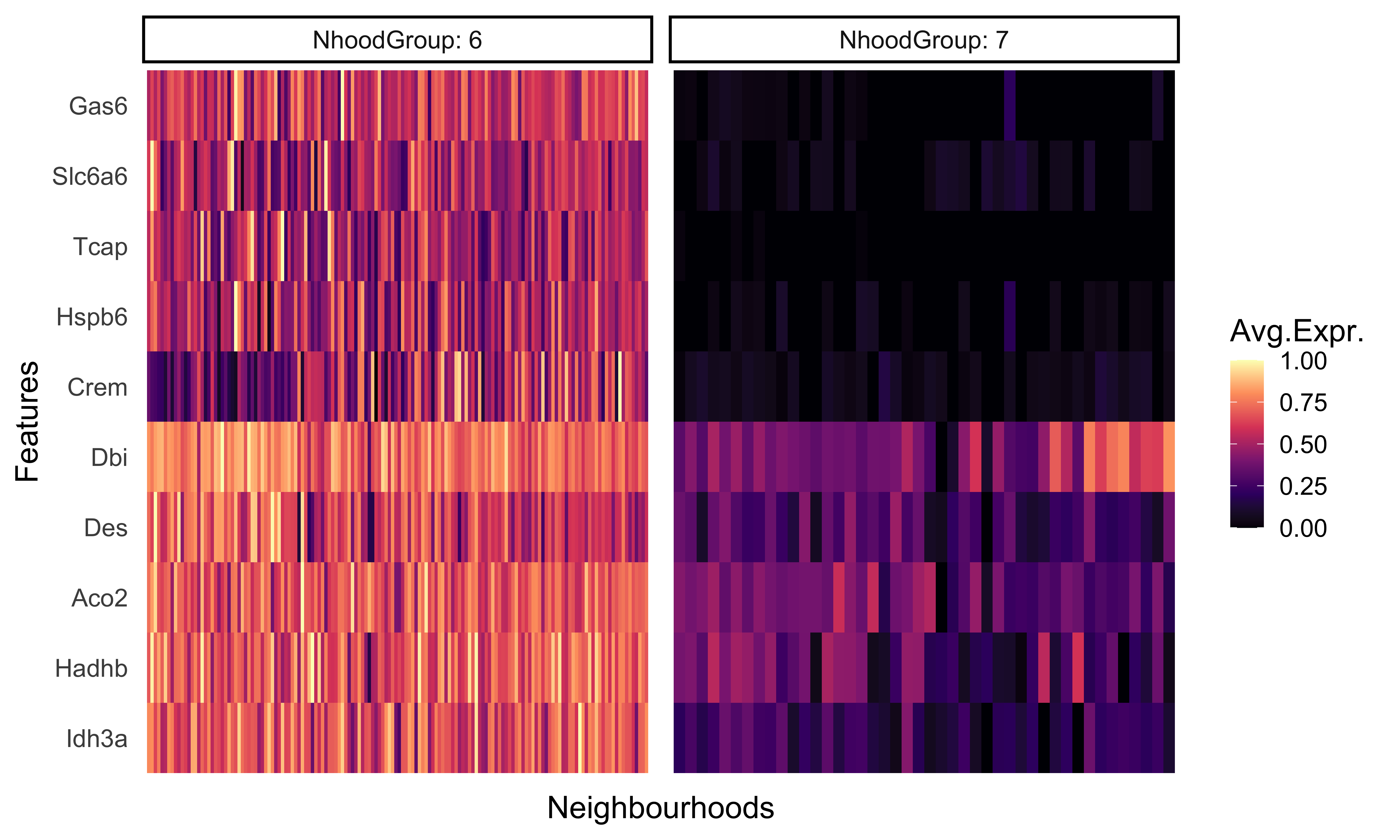
As we would expect, these genes are much more highly expressed in group 6 compared to group 7. This provides us with another, cluster-free alternative to looking at more subtle shifts in gene expression across smaller groups.
Now that you have reached the end of your analysis, make sure to output the versions of all tools used in the DE analysis:
sessionInfo()
For better reproducibility, it can help to create RMarkdown reports, which save all code, results, and visualizations as nicely formatted html reports. We have a very basic example of a report linked here. To create these reports we have additional materials available.
This lesson has been developed by members of the teaching team at the Harvard Chan Bioinformatics Core (HBC). These are open access materials distributed under the terms of the Creative Commons Attribution license (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.